Introduction
Diacylglycerol acyltransferase (DGAT) is a membrane protein that synthesizes triacylglycerides from its two substrates diacylglycerol (DAG) and fatty acyl-CoA for dietary fat absorption and fat storage. DGAT can be found expressed in the small intestine’s epithelial cells, in the liver where it synthesizes fat for storage, and in the female mammary glands where it produces fat for milk [1]. DGAT is a member of the membrane-bound O- acyltransferases (MBOAT) family [2]. All of the enzymes within this family are transmembrane enzymes that acylate lipids or proteins. Additionally, MBOAT enzymes have a conserved MBOAT core, a channel-like region that acts as the enzyme’s active site. Another feature of note within this MBOAT core is the conserved catalytic Histidine.
DGAT is a dimer that has two identical subunits. Each of the individual subunits contains an MBOAT core that acts as its active site. Each subunit also contains nine transmembrane alpha-helices (TM), 2 intracellular loops (IL), and one ER luminal loop (EL). TM2-9, IL1, and IL2 form the structure of the MBOAT core active site.
Function
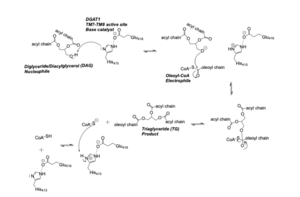
DGAT synthesizes triacylglycerides from diacylglycerol (DAG) and fatty acyl-CoA. The catalytic mechanism for triacylglyceride synthesis by DGAT is shown in Figure 1.
Structure
Dimer Interface
Active Site
The active site of DGAT serves its catalytic function by placing the His415 residue in close proximity to the acyl-CoA in order to cleave its ester bond and thus bind the fatty acid to the diacylglycerol. The conserved His415 is able to act catalytically due to a charge relay system, where the neighboring Glu416, due to its negative charge pulls electrons on histidine at the N1 position, making the N3 position more nucleophilic. This nitrogen will then deprotonate DAG so it can begin its attack on Acyl-CoA through acyl substitution.
DAG Binding
Acyl-CoA Binding
Disease
Relevance
[2]
[1]