Introduction
ACAT is a [tetramer] composed of a dimer of dimers.
Mechanism
Tunnels
The catalytic site is accessed through three different tunnels that lead from the center catalytic domain of the monomer, to the [lumen], cytosol, and transmembrane space. The tunnels allow the entrance of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function.
The is open to the cytosolic side of the protein in which the Acyl CoA enters into the catalytic domain.
The catalytic site contains conserved that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid tail.
The is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important of the T tunnel include Arginine262, Phenylalanine 263, and Leucine 306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism.
The is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel.
The mechanism of the [acyltransferace]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure X). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol.
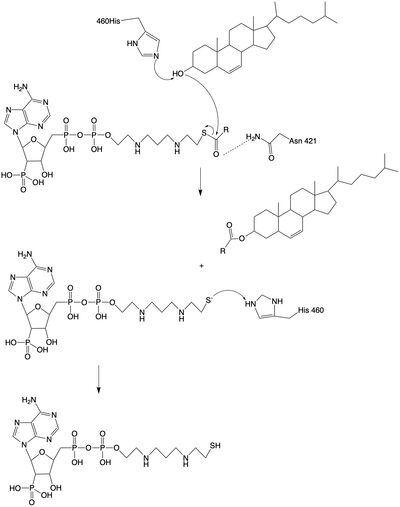
Figure Legend: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.
Crystal Structure of the Entamoeba histolytica RNA lariat debranching enzyme. [1]
Function
ACAT article [2]
SOAT Article [3]
Disease
Relevance
Structural highlights
The that constitute the T tunnel are critical to the activity of ACAT1.

