Introduction
Acyl-Coenzyme A Cholesterol Acyltransferase (ACAT), or also known as Sterol O-Acyltransferase (SOAT), is an important enzyme in the body.
Cholesterol esters were found in arterial lesions in 1910, but the first ACAT activity was discovered in the mid 1900's. This led to the inhibition of ACAT as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT inhibitors grew, but some of the compounds looked at exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT gene was successfully cloned. This discovery led to more studies with ACAT and atherosclerosis. Some of these studies used mice and showed cellular toxicity. ACAT inhibition is still being looked into as a strategy for treatment or prevention of atherosclerosis and related diseases.
[1]

Figure 1. ACAT as a Dimer of Dimers - One Monomer is Highlighted
Function
ACAT is an important enzyme that catalyzes the esterification of cholesterol to form cholesterol esters, and it belongs to the class of enzymes called acyltransferases. It is also a member of the MBOAT family because it is key in lipid metabolism. This enzyme is biologically important because it affects the solubility of cholesterol in the cell membrane and promotes accumulation of cholesterol ester in the cytoplasm as fat droplets. Accumulation of cholesterol ester as these lipid droplets is a main characteristic of macrophage foaming, which can lead to atherosclerotic diseases [2].
Structural Overview
ACAT is a dimer of dimers, which is also known as a tetramer.
This is about 260 kDa and is composed completely of helices, with each monomer containing 9 transmembrane helices, which have been color-coordinated to help with orientation within structures. The was found to be the active arrangement.
The is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway. The cholesterol enters through the T tunnel while the acyl-CoA enters through the C tunnel. The reaction is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol from the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen.
Subunits
ACAT is comprised of 4 subunits (A,B,C,D) to make up the tetramer. Two subunits make up the dimer (A,B and C,D) which is the form of ACAT which is the most active compared to the monomer and tetramer.
Domains
Tunnels
The catalytic site is accessed through three different tunnels that lead from the center catalytic domain of the monomer, to the [lumen], cytosol, and transmembrane space. The tunnels allow the entrance of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function.
The is open to the cytosolic side of the protein in which the Acyl CoA enters into the catalytic domain.
The is the transmembrane tunnel in which the cholesterol enters into the catalytic domain space. Important of the T tunnel include Arginine262, Phenylalanine 263, and Leucine 306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism.
The is used for the cholesterol ester product to be able to leave the lumen of the cell yet this exit mechanism is still unknown in addition to the cholesterol leaving to the transmembrane space through the T tunnel.
Active Site
The catalytic site contains that are essential to the mechanism of the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl CoA ligand is the presence of hydrophobic residues to stabilize the fatty acid (Trp407,Trp420). The active site is at the intersection of all three tunnels to allow a central position for the acyltransferase to occur. The H460 is positioned to deprotonate the cholesterol upon entering through the T tunnel: Acyl CoA upon entering is positioned to where the sulfur bonded to the carboxyl carbon is at the direct intersection of the T tunnel into the active site.
Mechanism
The mechanism of the [acyltransferace]reaction occurs in the catalytic site one of the monomers in the dimer of ACAT1. The T tunnel and and C tunnel converge to the same space to allow the proper orientation of the Acyl CoA and the incoming cholesterol from the transmembrane. The Acyl CoA is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (figure 2). This mechanism produced Acyl-CoASH and cholesteryl ester. The Acyl-CcASH leaves through the C tunnel to the cytosol.
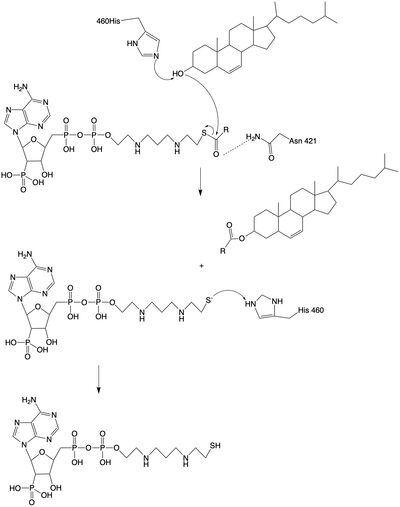
Figure 2: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.
Inhibitor
The illustrates the competitive inhibition of Acetyl-CoA and the inhibitor CI-976. According to Guan and colleagues, there have been a number of iterations of ACAT inhibitors have been created, and many of them of different structure formations [3]. CI-976 specifically is known as a small molecule inhibitor that is part of what is called the fatty acyl amide analog family, and functions as a competitive inhibitor of Acyl Co-A [3]. Guan discussed that this inhibitor in previous studies had shown that CI-976 reduced the size of atherosclerotic plaques and cholesterol levels in plasma [3]
Structurally, Acetyl-CoA and CI-976 are both largely hydrophobic, each with long hydrophobic tails and aromatic heads. As evident in this image, the hydrophobic tail of CI-976, mimics that of Acetyl-CoA. This allows for the inhibitor to be recognized by ACAT and to bind tightly in the active site pocket, blocking Acetyl-CoA from binding, thus rendering ACAT unable to perform its reaction.
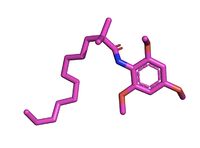
Figure 3. CI-976 Inhibitor
Disease Implications
Alzheimer's Disease
Alzheimer's Diseaseis a neurodegenerative disease characterized by accumulation of extracellular plaques that cause interferences with memory retrieval. These plaques are made up of amyloid beta (Aβ) peptides which are products of the cleavage of human Amyloid Precursor Protein (hAPP) [4] [5]. Within the cells, there is an accumulation of hyperphosphorylated tau [4] [5]. Research has shown that the concentration of cholesterol within cells can affect the production of Aβ [4] [5]. As the concentration of cholesterol in the endoplasmic reticulum of neurons increases, hAPP is downregulated [4][5]. Inhibition of ACAT1 would lead to higher concentrations of cholesterol in the cells, signaling downregulation of hAPP. Less hAPP available decreases the amount of Aβ peptides being produced and decreases the available Aβ peptides that could form the extracellular plaques associated with Alzheimer's Disease [4] [5].
Other Diseases
ACAT is also involved in diseases such as Parkinson’s Disease and other neurodegenerative diseases due to the accumulation of Aβ plaques in the brain. After research on glioma, prostate cancer, pancreatic cancer, leukemia, and breast cancer, it has been noted that ACAT plays a role in the progression of cancer over time. Recently, Ayyagari et al. found that there was a significant increase in ACAT-1 expression in ovarian cancer cell lines [6]. ACAT-2 is believed to be upregulated in Nephrotic Syndrome (NS) which can lead to cardiovascular disease and renal diseases [7]. Because of ACAT's activity in tissues such as the aorta, intestine, and liver, it plays a role in Atherosclerosis [8]. Studies have shown that the inhibition of ACAT-2 can slow the progression of Atherosclerosis [8]. Guan discussed a previous study which found that CI-976 decreased the size of atherosclerosis plaques and the overall concentration of cholesterol in the blood plasma of animals that had been fed a high cholesterol diet [3].



