User:Giselle Flores/Sandbox 1
From Proteopedia
Lipoprotein Lipase coupled with GPIHBP1
Introduction(LPL) is an important enzyme for the breakdown of triglycerides in the body (Figure 1).[1] A lipase is an enzyme that is capable of catalyzing the hydrolysis of fats/lipids which are consumed through oils. It is encoded by the p22 region in chromosome 8. Once synthesized, it is secreted into the interstitial space in several tissues. The main site of action for is in the capillary lumen within muscle and adipose tissues.[2] The function of this lipase is to hydrolyze triglycerides of very-low-density lipoproteins (VLDL) and to aid in the delivery of lipid nutrients to vital tissues.Cite error: Invalid<ref> tag;
refs with no content must have a name The enzyme is commonly found on the surface of cells that line blood capillaries. Two different lipoproteins are essential to break down triglycerides. One of the lipoproteins is utilized to transport fat into the bloodstream from different organs.Cite error: Invalid<ref> tag;
refs with no content must have a name The lipoproteins essential, in the transport of fat from the intestine are referred to as chylomicrons. VLDL are utilized in carrying triglycerides from the liver into the bloodstream. The hydrolysis of triglycerides by lipoprotein lipase results in fat molecules being used by the body as energy or stored in fatty tissue. Cite error: Invalid <ref> tag;
refs with no content must have a name[3]
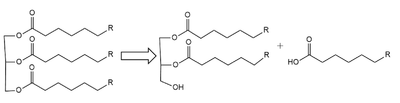
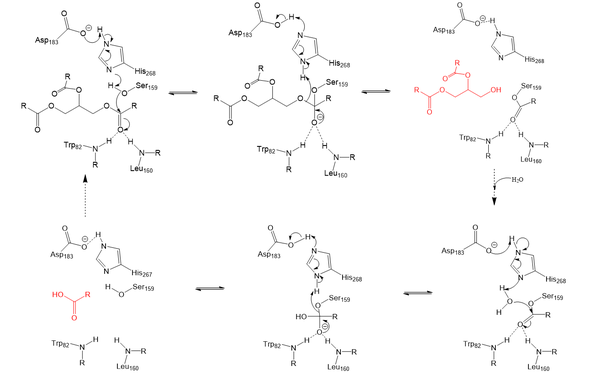
Structural OverviewLPL is assumed to only be active as a composed of two LPL-GPIHBP1 heterodimers, however, previous studies have argued that the lipase can be active in its .Cite error: Invalid GPIHBP1Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) is a secondary domain that is critical to the stabilization, function, and movement of .Cite error: Invalid Structural HighlightsCalcium Ion StabilizationIons are widely used in proteins and mechanistic stabilization in many areas of biochemistry. LPL’s tertiary folding is stabilized by a Calcium (Ca2+) ion. The calcium ion shares electron density with surrounding residues in order to orient the protein in its formal state.Cite error: Invalid Lid and Lipid Binding RegionIn the presence of the GPIHBP1 inhibitor, the and become visible within the structure. As displayed through a study conducted by Arora et. al, in 2019, the lipid-binding region of LPL actively interacts with the known inhibitor in the heterodimeric form. Cite error: Invalid MechanismLipoprotein Lipase functions to catalyze the hydrolysis of one ester bond of triglycerides in order to remove one fatty acid tail and turn the triglyceride into a diglyceride. It does this by utilizing a simple serine hydrolase mechanism, in which it uses a composed of Asp183, His268, and Ser159 to catalyze the hydrolysis. His268 serves as a base catalyst by deprotonation of Ser159, which can then serve as the nucleophile to attack the carbonyl carbon of one of the fatty acid chains of a triglyceride. This forms a tetrahedral intermediate, which is stabilized by the amide of Trp82 and Leu160 residues, called the . The single fatty acid chain is cleaved from the triglyceride, forming the diglyceride product. Then, water is used to free the fatty acid from Ser159 and return all of the residues back to their starting point, in order to perform the mechanism again on another triglyceride. Ultimately, the hydrolysis results in the formation of one free fatty acid and glycerol with two fatty acid tails (Figure 2). Relevance & DiseaseLPL is an extremely important enzyme, in that it is responsible for the proper breakdown of certain fats in the body. LPL breaks down triglycerides carried in chylomicrons, otherwise known as very-low-density lipoproteins (VLDL). Chylomicrons carry digested fats in the form of triglycerides out of the small intestine and into the bloodstream. LPL recognizes the chylomicrons, and hydrolyzes the associated triglycerides.[5] When triglycerides are not broken down properly and are allowed to build up, they can lead to increased plasma triglyceride levels (hypertriglyceridemia) and cholesterol buildup. Hypertriglyceridemia is very unhealthy and is the leading cause of Coronary Artery Disease in America.[6] Cholesterol buildup is caused by excess fats (triglycerides) and is a similarly serious issue with regards to obesity and heart disease in the United States as it can lead to plaque buildup in arteries and veins, which restricts blood flow.[7] Chylomicronemia, which is defined as an excess of chylomicrons in the blood, is the disease characterized by the body being deficient in LPL resulting in persistent hypertriglyceridemia. This disease causes the body to be unable to digest very much ingested fats and often leads to severe abdominal discomfort and several episodes of acute pancreatitis.[8] In short, without LPL in the body, triglycerides are unable to get broken down, and there is a much higher likelihood of developing coronary & metabolic based diseases. M404 MutationA 2018 study [9] argued that mutations that inhibit the proper binding of GPIHBP1 and LPL are suspected to cause chylomicronemia. A mutation that is believed to cause chylomicronemia is known as . Within this mutation, the methionine is replaced with a large and hydrophilic arginine. While this missense mutation does not impact LPL secretion, it does affect the formation of the LPL-GPIHBP1 complex by disrupting LPL’s interaction with GPIHB1’s Val121, Glu122, Thr124, and Val126 residues. This disrupts the stabilization of the LPL-GPIHBP1 complex, which has been seen to negatively affect LPL’s ability to catalyze the hydrolysis of triglycerides.
References
Student ContributorsGiselle Flores Dustin Soe Maggie Stopa | ||||||||||||