Microcrystal preparation for serial femtosecond X-ray crystallography of bacterial copper amine oxidase
Takeshi Murakawa, Mamoru Suzuki, Toshi Arima, Michihiro Sugahara, Tomoyuki
Tanaka, Rie Tanaka, So Iwata, Eriko Nango, Kensuke Tono, Hideyuki Hayashi, Kenji Fukui, Takato Yano, Katsuyuki Tanizawa, and Toshihide Okajima [1]
Molecular Tour
Visualization of enzymatic catalysis as molecular movies may be an ultimate goal for enzymologists. However, the direct observation of structural changes associated with the ongoing reaction has been a difficult task until recently. A new method called ‘Serial Femtosecond Crystallography (SFX)’, in which protein microcrystals delivered continuously are irradiated with ultrahigh-intensity X-ray pulses produced from X-ray free-electron lasers (XFELs), is expected to be a clue for achieving such a goal. Because diffraction data can be collected before the crystals suffer from radiation damage, SFX allows the determination of X-ray damage-free structures at room temperature. The femtosecond duration of X-ray pulses is also advantageous for visualizing rapid structural changes associated with protein action. However, preparation of high-quality microcrystals with uniform size has been a bottleneck for widespread application of SFX that consumes plenty of microcrystals. This paper reports a convenient method for production of uniform, high-quality microcrystals by combining micro-seeding and batch methods. The objective protein we used is a quinoprotein metalloenzyme, copper amine oxidase from Arthrobacter globiformis (AGAO), for which high-resolution X-ray and neutron crystal structures have already been determined at cryogenic temperatures. The first X-ray-damage-free AGAO structure determined at room temperature by SFX (PDB ID: 7f8k) is herein reported.
The overall structure of AGAO determined by SFX had almost the same conformation as those determined previously by synchrotron X-ray and neutron crystallography performed at cryogenic temperature. The electron-density map in the active site clearly showed the resting form of the protein-derived cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ). In addition, the active-site Cu2+ was ligated with three histidine residues and two water molecules that are located at positions identical to those determined by the previous studies. These results show that the bound Cu2+ in AGAO is resistant to X-ray photoreduction, which is accompanied by conformational changes of the metal coordination structure. The availability of high-quality microcrystals in large quantities is promising for studying the structural changes of AGAO during the catalytic reaction by the ‘mix-and-inject’ time-resolved SFX.
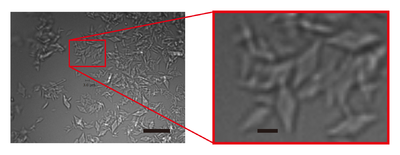
Figure 1 Images of AGAO microcrystals grown by combining micro-seeding and batch crystallisation. The right panel shows an enlarged view of the part indicated by a red rectangle in the left panel. Scale bars in the left and right panels represent 20 and 3 µm, respectively.
References
- ↑ Murakawa T, Suzuki M, Arima T, Sugahara M, Tanaka T, Tanaka R, Iwata S, Nango E, Tono K, Hayashi H, Fukui K, Yano T, Tanizawa K, Okajima T. Microcrystal preparation for serial femtosecond X-ray crystallography of bacterial copper amine oxidase. Acta Crystallogr F Struct Biol Commun. 2021 Oct 1;77(Pt 10):356-363. doi:, 10.1107/S2053230X21008967. Epub 2021 Sep 21. PMID:34605440 doi:http://dx.doi.org/10.1107/S2053230X21008967


