Fel d 1
From Proteopedia
1PUO - Fel d 1: The major cat allergen
The major allergen in cats, Fel d 1, belongs to the secretoglobin family of proteins and is, worldwide, one of the major causes of allergic asthma, as it induces IgE responses in 90 to 95% of those allergic to cats and in 60 to 90% of total allergenic activity to cat hair. Symptoms range from mild rhinitis and conjunctivitis to life-threatening asthmatic responses. The structure of Fel d 1 displays the location of three previously defined Fel d 1 IgE epitopes on the surface of the protein [1]. It is produced mainly by the salivary and sebaceous glands, it is also present in the perianal, lacrimal and squamous epithelial cells and is attached to the cat's hair through the habit of licking [1][2].
FunctionThe biological function of Fel d 1 is still unknown, however it is thought to have a pheromone/chemical signaling function[2]. StructureFel d 1 is a tetrameric glycoprotein of 35 to 39 kDa, by size exclusion chromatography, formed by two identical heterodimers of about 18 kDa, noncovalently linked. These heterodimers are totally α-helical, formed by 8 helices, H1-H4 and H5-H8, corresponding to the 2 and 1 chains, respectively[1][3]. Chains 1 and 2 are two antiparallel polypeptides, linked via 3 interchain disulfide bonds, formed between cysteine residues at positions Cys3-Cys73, Cys44-Cys48, and Cys70-Cys7, at chains 1 and 2, respectively, in chains 1 and 2, respectively[1][3]. 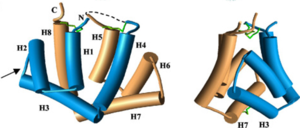 Figure 1. General structure of a Fel d 1 monomer, shown in two distinct orientations, rotated 90° about the vertical axis[1]. Chain 1 has about 8kDa, composed of a residue of 70 amino acids and chain 2 has about 10 kDa, which can be composed of a residue of 90 amino acids, found preferably in the sebaceous glands, or composed of a residue of 92 amino acids, which is expressed by the salivary glands. Its glycan portion is found in chain 2 and the recombinant structure of Fel d 1 reveals that the N33 residue is located in the loop connecting the H2 and H3 helices and that the side chain is exposed to the solvent[1][3]. 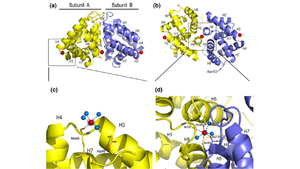 Figure 2. General structure of the Fel d 1 tetramer. (a) Schematic view of the Fel d 1 tetramer (1 + 2). The two heterodimeric subunits A and B, each composed of the linked 1 and 2 chains, that form the tetramer are yellow and blue, respectively. The three Ca2+ ions are indicated as red balls. (b) Schematic view of the Fel d 1 tetramer following an approximately 90° rotation about the horizontal axis. (c) Calcium binding sites 1 and 2. (d) Calcium binding site 3[4]. Figure 1 shows the general structure of a Fel d 1 monomer, shown in two different orientations, rotated 90° around the vertical axis. Chains 1 and 2 correspond to the gold and blue helices respectively. The dotted line indicates the disordered loop (residues 75 to 92). The three disulfide bridges connecting chains 1 and 2 are shown in green. An arrow indicates the unique glycosylation site at residue N33[1]. 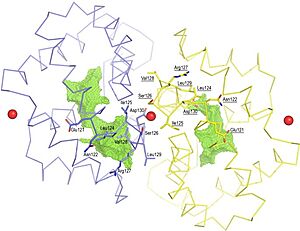 Figure 3. Shape of the cavities (in green) directly governed by the conformation of Leu129 and Asp130 residues. The Asp130 side chain (underlined) does not interact with Ca2+ and projects into the cavity in the A subunit (in yellow), while the Asp130 side chain (B subunit) binds to Ca2+. The two external Ca2+ ions are also indicated[4]. In the Fel d 1 tetramer, three Ca2+ binding sites were identified[3], as in Figure 2, where the Ca2+ are indicated as red balls. Two Ca2+ binding sites are equivalent and are found symmetrically located on either side of the dimer and the third is found within the dimerization interface (Figure 2 (a) and (b)). The equivalent Ca2+ binds to the carbonyl groups of residues Asp46 and Met49, as well as to four and three water molecules in the A and B subunits, respectively (Figure 2 (c)) and the Ca2+ located at the dimerization interface binds to the OD1 atoms of residues Asn89 (in subunit A), Asn89 (B) and Asp130 (B), as well as to the carbonyl group of residue Ile125 (A) and three molecules of water (Figure 2 (d))[4]. Furthermore, the Fel d 1 quaternary structure reports two cavities in the A and B subunits of 350 and 730 Å3, respectively, where the difference in size between the two cavities is a direct result of the conformational change within the region corresponding to residues 121– 131. This size difference is related to the conformation of the residues Leu129 and Asp130, which point in opposite directions in the A and B subunits. Furthermore, the Ca2+ from the dimerization interface does not interact with the side chain of the Asp130 from the A subunit, projecting itself, then into its cavity, while the Asp130 side chain of the B subunit interacts with Ca2+. Such cavities have 3 and 7 water molecules, respectively. All of this can be seen in Figure 3[4]. In the 3D scene it is possible to by group and make of the protein Fel d 1 [5] [6]. Treatment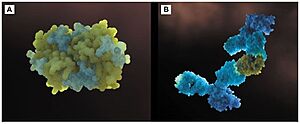 Figure 4. (a) Three-dimensional configuration of Fel d 1 (b) Fel d 1 linked to two anti-Fel d 1 IgY antibodies [2]. Immunotherapy, or allergy vaccination, which is based on repeated subcutaneous injections of cat hair extracts has been shown to be effective in the curative treatment of allergy. However, in addition to being a time-consuming treatment, it can cause serious side effects such as asthma attacks and anaphylactic shock [1][3]. Additionally, to add to the immunotherapy treatment, there is a cat food supplemented with anti-Fel d 1 IgY, which significantly reduces active Fel d 1 levels. Figure 4 shows the three-dimensional configuration of Fel d 1 (a) and the structure of Fel d 1 linked to two anti-Fel d 1 IgY antibodies (b) [2]. References
| ||||||||||||
