This is a default text for your page Natalia de Araujo./Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
The first structure, displayed on the left-hand side of the screen, corresponds to 4jon, the Crystal structure of CEP170's transcript variant beta, from Homo sapiens. 4jon is a 5 chain structure obtained by X-ray Crystallography [1] and represents the first 126 residues of CEP170's N-termini region.
The second structure available for CEP170 is , a predicted 3D model representing its full lenght (1-1584).
Here we are going to focus on the Q5SW79 model due to its full sequence, allowing us to highlight residues and regions important for the understanding of CEP170's functions and interactions.
|
|
| Available 3D models: | 4jon, |
| Specie: | Human |
| Gene: | CEP170, FAM68A, KAB, KIAA0470, NM_001042404 (HUMAN) |
| Splice Variants | CEP170α, CEP170β, and CEP170γ |
| RefSeq (mRNA): | CEP170α: NM_014812[2], CEP170β: NM_001042404 [3], CEP170γ: NM_001042405[4] |
| RefSeq (protein): | CEP170α: NP_055627[5], CEP170β: NP_001035863[6], CEP170γ: NP_001035864[7] |
| UniProt: | Q5SW79[8] |
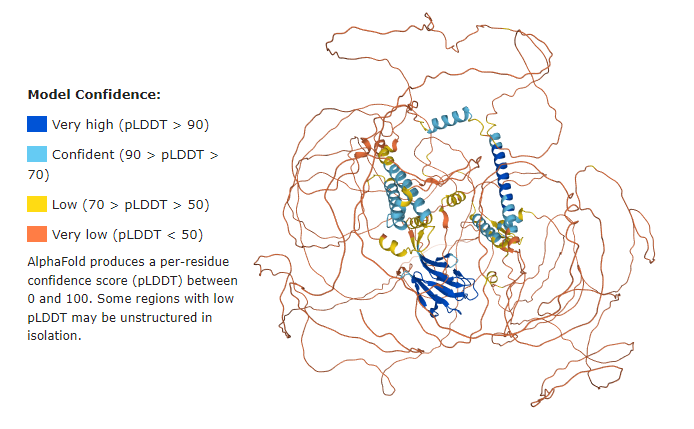
Q5SW79 Confidence Model
Before getting started, it is important to have in mind that the majority of the Q5SW79 structure has very low confidence, meaning the model most probably does not represent the real protein structure.
However, it stills helps us to have a good idea of the whole protein.
See below the confidence model from UniProt:
CEP170 gene
The CEP170 gene (OMIM*613023[9]) spans over 131,369 bases at 1q43, contains 30 exons, and encodes the Centrosomal Protein of 170 kDa (CEP170)[10].
Bulk RNA-seq of CEP170 [11](average expression of the gene) shows that CEP170 is expressed in a variety of tissues.
CEP170 protein
CEP170 is a pericentriolar protein that associates with the mother-centriole during interphase and with the spindle apparatus during mitosis[3], showing a dynamic behavior throughout the cell cycle.
CEP170 plays an important role in microtubule organization, microtubule nucleation, and the proper dynamics of the mitotic spindle.
Centrosome, Centriole, and Spindle pole.
At the centrosome, CEP170 binds specifically to the subdistal appendages of the mother-centriole.
Protein-Protein Interactions
- CHEK1 and circCHEK1_246aa
Through Co-IP [12]and MS[13] CEP170 was identified as a putative interactor with CHEK1, further confirmed with Co-IP in cells overexpressing CHEK1 [4]
CHEK and circCHEK1_246aa (isoform from a cRNA) phosphorylate the residue of CEP170, which results in chromosomal instability seen in multiple myeloid cells.
Through yeast two-hybrid screen CEP170 was identified as a putative interactor with PLK1, further confirmed with Reciprocal Co-IP in vivo.
PLK1 phosphorylates the C-termini region () of CEP170. [5]
Through affinity purifications of GFP-Kifc3 CEP170 was identified and confirmed as a KIF2B interactor.[6]
Proximity ligation assay (PLA) showed co-localization of TBK1 and CEP170 and the interaction was further confirmed with Co-IP experiments.
In vitro kinase assays showed that TBK1 phosphorylates 12 serine residues and 1 threonine residue of CEP170 (see phosphorylation).[7]
CCDC120 co-fractionated with CEP170 and interaction was confirmed with immunoprecipitation. CCDC120 binds to the the C terminus() of CEP170.
to CCDC120.
Through MS CCDC68 was identified as a CEP170 interactor, further confirmed by immunoprecipitation.[8]
Through proximity-dependent biotin identification (BioID) and MS CEP170 was identified in possible interaction with CCDC61, further confirmed with immunoprecipitation.
CCDC61 plays a role as a mediator of the interaction between TBK1 and CEP170.[9]
Though Co-IP and iTRAQ MS CEP170 was identified as a putative interactor with WDR622, further confirmed with Co-IP experiment in cells overexpressing WDR62 or CEP170.[10]
Structural highlights
As seen by Guarguaglini et al. (2005) all three splice variants harbor an N-terminal forkhead-associated (FHA) domain and a serine-rich domain and a short coiled-coil region in the C-terminal half.
The C-terminus region harbors 3 globular domains, [.......] located at and the second at
As shown previously, CEP170 is a phosphoprotein, phosphorylated probably by PLK1, TBK1, CHEK1 and circCHEK1_246aa and [...]
The serine-rich region is located at x-x, harboring around 48 phosphorylated residues
|
|
| Phosphoserine: | ; ; 356; 359; 446; 466; 497; 571; 580; 630; 633; 636; 667; 725; 838; 879; 881; 930; 933; 958; 1019; 1059; 1112; 1114; 1132; 1133; 1145; 1160; 1165; 1198; 1205; 1210; 1239; 1241; 1251; 1270; 1280; 1362; 1521; 1522 |
| Phosphotyrosine: | 364 |
| Phosphothreonine: | 501; 667; 760; 914; 920; 1023; 1058 |
| Mashup: | |
| Proteins: | PLK1, blabla |
FHA N-terminal (22-73aa) iso alfa
- Serine-rich region 968-1228aa iso alfa
- Targeting to microtubules: 853 – 1584
- Interaction with CCDC61: 1015 – 1460
- Targeting to centrosomes:1113 – 1584
- Coiled coil domain:1467 – 1495(mesmo da guargua, iso alfa).. Protein which contains at least one coiled coil domain, a type of secondary structure composed of two or more alpha helices which entwine to form a cable structure. In proteins, the helical cables serve a mechanical role in forming stiff bundles of fibres. uniprot https://www.uniprot.org/keywords/KW-0175
Disease
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.