User:Julia Takuno Hespanhol/Sandbox 1
From Proteopedia
VRR-Nuc domain
scene=/>
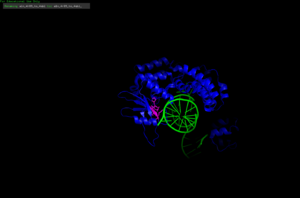
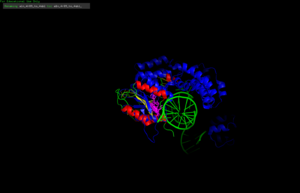
The use of the phage structure in Proteopedia [1]
IntroductionThe VRR-Nuc (Virus type replication repair nuclease) is a domain found in different enzymes of both eukaryotes and prokaryotes, including humans, bacteria and pages. The VRR-Nuc domain belongs to the phosphodiesterase superfamily PD(D/E)xK, which is a widespread family that contains nucleases with most diverse functions, including, but not restricted to, DNA repair and modification, restriction endonucleases, RNA modification, holiday junction resolvases and, most recently, bacterial antagonist effectors. [2][3] Structural HighlightsThe VRR-Nuc containing proteins, since belonging to the PD(D/E)xK superfamily, present structure similar to the core structure of the superfamily. The conserved core includes αβββαβ, in which the conserved residues responsible for the nuclease activity D (Aspartic Acid), D/E (Glutamic Acid), K (Lysine) are usually found in the second and third β-sheets. The Aspartic and Glutamic Acid residues coordinates metal atoms, while the Lysine residue associates with a water molecule to attack the phosphodiester bond. The α-helixes are usually associated to substrate binding and enzyme dimerization and stabilization. [2][3] The VRR-Nuc containing proteins might present a single domain, only the VRR-Nuc domain [1], or present other domains, such as the human FAN1 (human FANCD2-associasted nuclease) [4] and its homologues [5]. The FAN1 is composed by 4 well conserved domains: UBZ (ubiquitin-binding zinc finger), which participates in substrate recognition and binding; SAP (SAF-A/B, Acinus and PIAS) related to DNA binding; TRP (tetratricopeptide repeat) implicated in FAN1 dimerization and protein-protein interactions; and the VRR-Nuc catalytic domain. [4]
Enzymatic ActivitiesThe VRR-Nuc domain can cleave the phosphodiesters bonds from nucleic acids. The domain in the single domain enzymes, such as psNUC from Psychrobacter (PDB:4qlb), presents Holliday Junction Resolvase activity [1]. The psNUC acts as dimer over four-way DNA-junctions, cleaving the DNA double-strand [1]. FAN1, however, is not able to process Holliday junctions, but has been identified presenting exo and endonuclease activity on different DNA substrates, such as: 5’ flaps; nicked double-strands; interstrand crosslinked (ICL) DNA; and double strands [4].
Biological functions and Related PathogenesisOne of the first characterized VRR-Nuc containing proteins is the human FAN1. This enzyme is though to belong to a pathway of ICL DNA repair, which is fundamental for cell DNA integrity [4]. ICLs may occur from exposure of mutagenetic compound in the environment, such as chemotherapeutic treatments [6]. The ICLs are highly toxic since they hinder protein translation by preventing DNA strands separation [6]. FAN1 is capable of finding ICLs in the double stranded DNA and unhook the crossliked DNA, reaching the target by exo and endonuclease activity by the VRR-Nuc domain [4].
Fanconi Anemia (FA) is a genetic disorder caused by defects in one of the genes from the ICL repair pathway. The FAN1 was though to belong to this pathway for its direct interaction with the FANCD-2 protein, which coordinates proteins from the pathway. However, studies indicate that FAN1 acts independently of this pathway [4]. FAN1 defect leads to greater sensibility to ICL inducing agents, also confirming the enzyme in the ICL repair [4]. Although there is not a specific disease associated to mutation in the FAN1 protein, mutations that lead to a truncated protein in the nuclease domain have been correlated to KIN (Karyomegalic interstitial nephritis), that leads to chronic interstitial nephropathy [4] Other disturbs related to FAN1 mutations are cancer, autism spectrum and schizophrenia. [4] FAN1 could even be related to cancer resistance to treatment, or be used as potential biomarker for eminent cancer resistance to drugs. [4]
References
| ||||||||||||||||||