Anaplastic Lymphoma Kinase is a receptor tyrosine kinase. ALK transfers a phosphate group from ATP to a tyrosine residue on an enzyme which activates a signaling cascade. ALK is an integral membrane protein.
Function
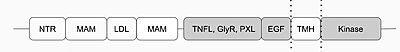
Fig.1 Anaplastic Lymphoma Kinase and its domains. The region from NTR to the MAM is the Heparin Binding Domain. The TNFL-PXL are the extracellular domains and the EGF is the domain that binds the extracellular region with the extracellular region of the transmembrane. The TMH is the transmembrane domain. The kinase domain is the intracellular portion of the ALK.
Conformational Change
In order for the Anaplastic Lymphoma Kinase (ALK) to move across the membrane to bind with the second monomer, it has to undergo a conformational change. The Anaplastic Lymphoma Kinase Activating Ligand (ALKAL) binds to the binding surface on the ALK. This induces a conformational change which allows for the PXL and the GlyR domains to hinge forward. The kinase is now ready to move across the membrane and attach to the other kinase to be fully activated.
Movement across the membrane
The negatively charged phosphate groups on the cell membrane interact with the highly conserved positively charged residues on ALKAL ligand that face the membrane, which stabilizes the ligand to bind to ALK even better.
Disease
Relevance & Role in the body
Structural highlights
The active state of the kinase is when two monomers have completed a conformational change and moved across the membrane to form a .

