This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
G-Protein Coupled Receptors
G-protein coupled receptors(GCPRs) are a large family of cell surface membrane proteins. Once bound to a wide variety of extracellular ligands, GCPRs undergo a conformational change and relay information to intracellular secondary messengers¹. This G protein activation results in a cellular response dependent on the ligand bound and location of the GPCR in the body. GCPRs can be broken down into five families: the rhodopsin family (class A, 701 members), the secretin family (class B, 15 members), the adhesion family (24 members), the glutamate family (class C, 15 members), and the frizzled/taste family (class F, 24 members)². All of the families have a similar transmembrane (TM) domain consisting of seven ɑ-helices complexed with intracellular G proteins.
Class A GCPRs
Class A GPCRS or rhodopsin-like GPCRS are the largest and most studied type of GPCRS. Due to the diversity of these receptors they are found in many aspects of physiology. They are characterized by conserved motifs including DRY, PIF, Sodium Binding, and the CWxP.6 A common example of a class A GPCRS is the β2AR receptor (PDB: 3sn6).
MRGPRs
The human itch GPCR, or Mas-related G-protein coupled receptor (MRGPR), is a Class A GPCR found in human sensory neurons and is responsible for the sensation of “itching” caused by skin irritation and diseases, insect bites, and hypersensitivity to certain drugs. There are currently four groups consisting of MRGPRX1, MRGPRX2, MRGPRX3, and MRGPRX4. In particular, MRGPRX4 is responsible for cholestatic itch while MRGPRX2 regulates degranulation and hypersensitivity itch reactions³. These two, chiefly MRGPRX2, are often targets for drugs that result in mast cell degranulation and hypersensitivity side effects. In comparison to other Class A GPCRs, MRGPRX2 binds to an even wider range of ligands, including agonists such as;
Structure with
Structure with
Types of Ligand Bound
Cation
Peptide
Specific Traits
Disulfide bonds
Toggle Switch
PIF Motif
DRY Motif
Sodium Binding
MRGPRX2 Signaling Pathway
Function
Conformational Changes
What It Triggers
Clinical Relevance
Many drugs have been linked to the activation of MRGPRX2 as a side effect that causes the sensation of itchiness. Among these drugs are morphine, codeine, and dextromethorphan. These drugs have a similar structure to that of (R)- ZINC-3573, introducing the idea of a similar binding mechanism.
Due to mutations in key structural features of typical Class A GPCRS, MRGPRX2, it is able to bind to a variety of substrates that then mediate the signaling pathway for the sensation of itching.
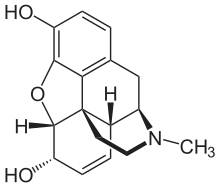
Figure X: Snake Plot of GCGR TMD. Residues of particular importance in glucagon binding affinity are found in green, yellow, and black. Residues in red are the location of critical disulfide bonds, while blue residues were found to be highly conserved across all class B GPCRs.
References
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.

