Anaplastic Lymphoma Kinase (ALK)
Introduction
Anaplastic lymphoma kinase is a receptor tyrosine kinase (RTK) that is important in regulating functions within the central nervous system [1]. The route to discovery of this protein's structure was rather complex, spanning almost 20 years; the kinase domain was discovered in 1994, the full protein structure in 1997, and the ligand structures in 2014. These structures were found using cryo-electron microscopy,nuclear magnetic resonance, and X-ray crystallography. Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer [2].
General Structure
Anaplastic lymphoma kinase is a , each monomer consisting of seven domains and two regions
[3]. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), (TNF), (GlyR), (EGF), transmembrane α-helix (TMH), kinase domain
[1]. The structures of the N-terminal region, MAM, and LDL have not been determined. The glycine rich region is a part of the TNF domain. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular domain.
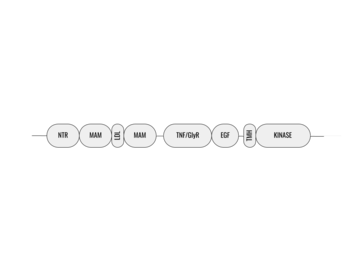
Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase
Ligand Binding
The ligands utilized by anaplastic lymphoma kinase are monomeric FAM150 and . It's biologically preferred ligand is dimeric AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane. Once the ligand is , ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane. These residues interact through the formation of . This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer.
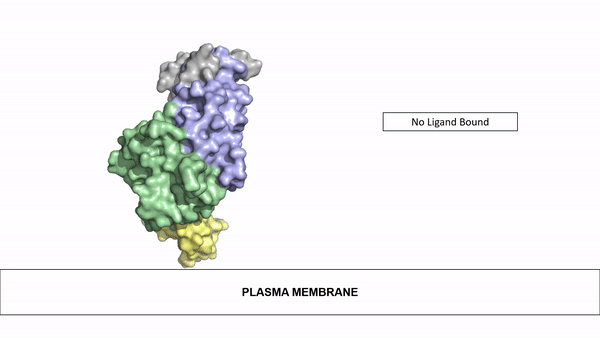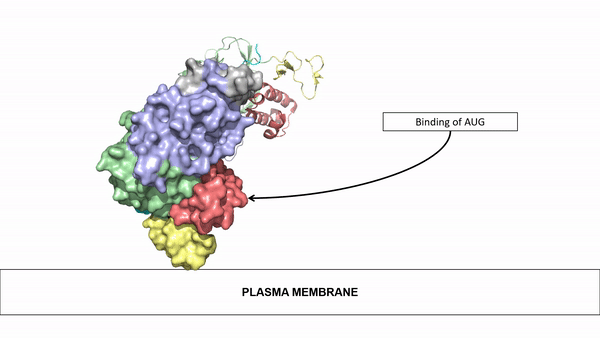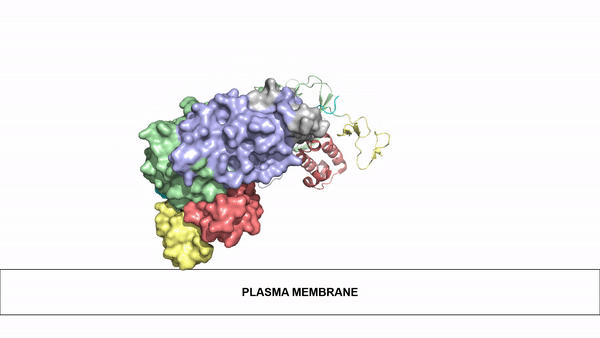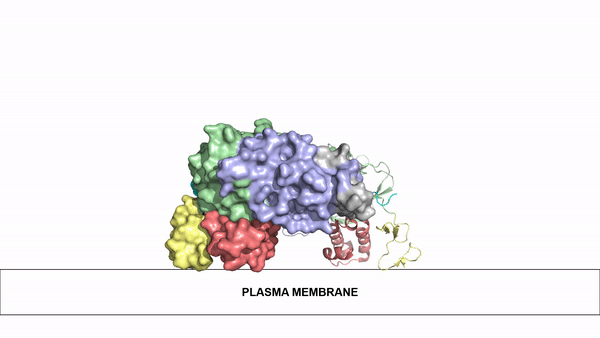
Figure 2: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.
Tyrosine Phosphorylation Mechanism
Dimerization of anaplastic lymphoma kinase activates the kinase domains of each monomer. Next, the kinase domains phosphorylate the tyrosine residues of the opposite monomer using ATP. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade through utilizing various signal pathways. These pathways signal for cell proliferation and survival (ex: begin transcription).
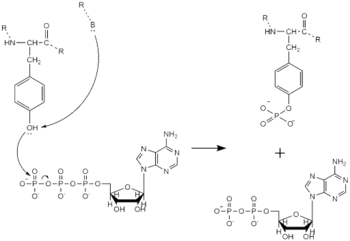
Figure 3. Tyrosine phosphorylation mechanism
Applications
Anaplastic lymphoma kinase's involvement as a proto-oncogene in various types of cancers has made it a target for drug therapies for these types of cancers [4]. One therapeutic medication that has been increasingly used with high levels of success is . This medication was approved by the FDA in January of 2021 for the treatment of pediatric/young adult ALK-positive anaplastic large cell lymphoma. The treatment has an overall response rate of 90%. Crizotinib works by to the ATP binding site of the [5]. Crizotinib binds preferentially to ATP in this region and therefore can effectively block the binding of ATP [5]. With the binding of ATP being blocked, the intercellular signaling cascade cannot begin which works to prevent the cancer from growing and spreading .



