User:Michael O'Shaughnessy/ TS
From Proteopedia
Contents |
Overview
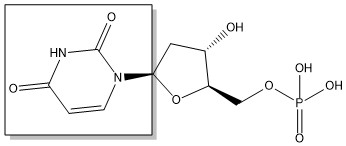
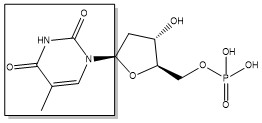
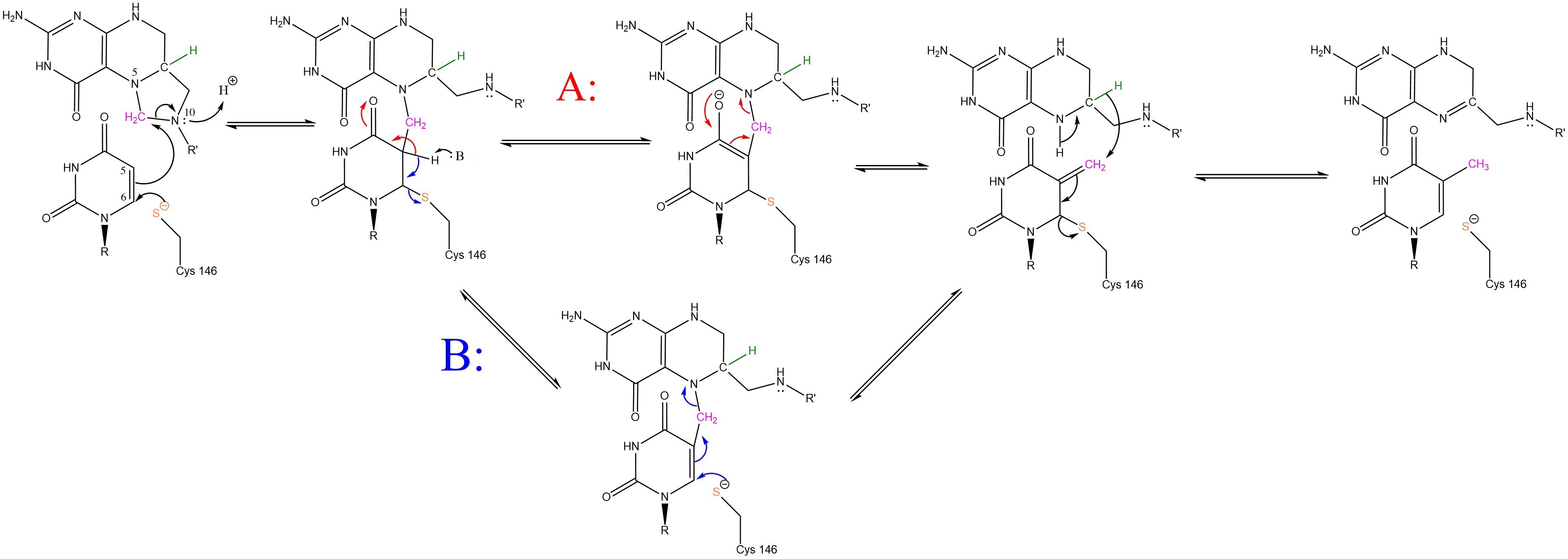
The enzyme Thymidylate Synthase (TS) catalyzes the transfer of a methyl group and a hydride from 5,10-methylenetetrahydrofolate to 2-deoxyuridine-5'-monophosphate, resulting in the formation of thymidine 5'-monophosphate and dihydrofolate. This is the only de novo source of dTMP in humans.
2-deoxyuridine-5'-monophosphate(dUMP) + 5, 10-methylenetetrahydrofolate(CH2H4F) ⇌ thymidine 5'-monophosphate(dTMP) + Dihydrofolate(H2F)
Function
Thymidyalte synthase has a critical role in cell division as the sole producer of thymine in the body.
Disease
Relevance
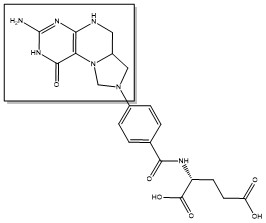
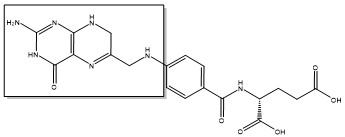
Due to its role in cell division, thymidylate synthase has become a popular target for anticancer drugs. Indirect inhibition of thymidylate synthase by the drug 5-fluorouracil (5-FU) is one of the most used inhibitors for study of TS function. This drug indirectly inhibits TS as it it eventually converted to FdUMP, which forms a covalent complex with both the active site cysteine and CH2H4F.
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ doi: https://dx.doi.org/10.1016/S1074-5521(01)00067-9