Introduction
Vitamin K epoxide reductase (VKOR) is the enzyme responsible for regenerating vitamin K from vitamin K epoxide to support blood coagulation.
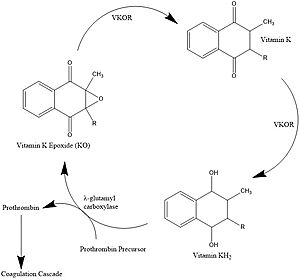
Figure 1. Vitamin K Cycle λ-glutamyl carboxylase uses Vitamin KH2 to carboxylate blood clotting cofactors, converting KH2 to KO in the process. Prothrombin is shown as an example of a blood clotting cofactor that later enters the coagulation cascade. VKOR converts KO back to KH2 via two steps with Vitamin K as an intermediate.
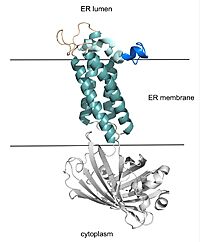
Figure 2. VKOR with Barrel Domain The teal helices are the transmembrane helices situated in the ER membrane. The tan sections are the beta loop and cap loop. The light blue section is the cap helix, and the dark blue section is the anchor. The cap loop, cap helix, and anchor make up the cap domain. The white barrel domain was used for stabilization during structural experiments and is not shown in future scenes on this page.
Vitamin K Cycle
Vitamin K is essential for blood clotting in the body[1]. The fully reduced form, KH2, is used by gamma-glutamyl carboxylase to carboxylate protein-bound glutamate residues in blood clotting cofactor precursors [2]. After carboxylation, the clotting cofactors (such as prothrombin) can bind to calcium and can proceed to the coagulation cascade [3]. During this process, KH2 becomes oxidized to Vitamin K epoxide, or KO [2]. Vitamin K epoxide reductase, abbreviated VKOR, turns the epoxide back to the fully reduced form so the reduced form can be used again. This transformation happens in two steps: 1) converting the epoxide to the partially oxidized Vitamin K quinone and 2) converting the quinone to the fully reduced hydroquinone (KH2) (Figure 1) [1].
Structural Overview
VKOR consists of four embedded in the endoplasmic reticulum membrane. The barrel domain was used experimentally to stabilize VKOR for structure determination (Figure 2)[4]. For this page, the barrel domain has been removed and structures renumbered to correspond with the article by Liu. [4]. Helices one and two are connected by the region which contains two of the active cysteines, C43 and C51; these cysteines, along with C132 and C135, are essential for reduction and structural changes discussed in the next section[4]. VKOR also has a covering the active site, made up of an , , and . The anchor serves to attach the cap domain to the ER membrane for stabilization[4]. The loop helps stabilize one of the catalytic amino acids, Asn80[4]. The helix is involved in stabilization of certain disulfide bonds and structural changes as part of the catalytic cycle discussed below[4].
Catalytic Cycle
Catalytic Cysteines
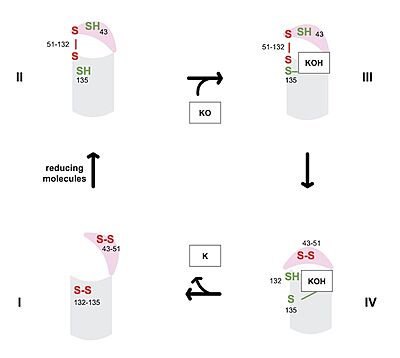
Figure 3. Catalytic Cycle of VKOR
The catalytic cycle of VKOR includes transitions from open to closed conformations by means of disulfide bridge-induced conformational changes. The substituent cysteines (43, 51, 132, and 135) also act as reducing agents for the substrate, which can be either Vitamin K epoxide (KO) or partially reduced Vitamin K. The first step of the catalytic cycle (Figure 3) is the wild type open conformation, . This step is characterized by an open cap domain with disulfide bonds between cysteines 43 and 51 and between cysteines 132 and 135. The second step of the catalytic cycle is a closed conformation, . This step is characterized by a disulfide bond between the cap domain and alpha helices (Cys51 and Cys132), with both containing an SH group. The next step of the cycle, , is also a closed structure with the same disulfide bond between Cys51 and Cys132. Cys135 is not involved in a disulfide bridge and assists with substrate binding. of the catalytic cycle is the last closed conformation. The Cys51-Cys132 bond is broken as Cys43 bonds with Cys51, recreating the disulfide bridge pattern of the open state. Cys132 is then free to bond with Cys135, releasing the product that was bound to the Cys135. The product will be either Vitamin K if the substrate was the epoxide or fully reduced Vitamin K hydroquinone if the substrate was Vitamin K. [4]
The anticoagulant warfarin works by inhibiting VKOR (See "Medical Relevance"). Warfarin binding also depends on the catalytic cysteines. Warfarin is able to bind to the fully oxidized open form of VKOR as shown in . Once Warfarin binds, VKOR is considered to be in a closed conformation since the substrate cannot enter, despite the lack of disulfide bridge changes. Warfarin can also bind to the partially oxidized form of VKOR as shown in .
Catalytic Amino Acids
VKOR uses two catalytic amino acids, tyrosine 139 and asparagine 80, to stabilize in all forms and , such as Warfarin, in the binding pocket. Tyr139 and Asn80 hydrogen bond to carbonyl groups on both structures and stabilizes them within the binding pocket.
Hydrophobic Interactions
Other than the two previously mentioned hydrogen bonds (Tyr139 and Asn80), and are bound in via hydrophobic interactions within the binding pocket of VKOR. Hydrophobic residues of VKOR such as Phe80, Phe87, and Tyr88, form a hydrophobic tunnel within the binding pocket.
Medical Relevance
Warfarin
Warfarin is the most widely prescribed oral anticoagulant, which targets vitamin K epoxide reductase. The FDA approves its use for cardiac conditions (myocardial infarction, atrial fibrillation) as well as for deep vein thrombosis and pulmonary embolism. Due to the inhibition of the normal blood clotting cycle, patients taking warfarin are at risk for hemorrhage which can occur anywhere in the body. [5]
Warfarin is a of Vitamin K and acts as a competitive inhibitor. There are around 30 known missense mutations that lead to warfarin resistance in patients, but these mutations do not affect Vitamin K binding for reasons which are not yet fully understood. Such patients require higher doses of warfarin to reach therapeutic level or require a different anticoagulant drug. [6]
Superwarfarins

Figure 4. Warfarin and Brodifacoum
More potent warfarin derivatives, called superwarfarins, are used as rodenticides. Superwarfarins have bulkier side chains that allow them to stay bound to VKOR for long periods of time, causing prolonged and uncontrolled bleeding. The duration of one superwarfarin, brodifacoum, has been reported as 15-30 days [7] vs. the clinical warfarin duration of 2-5 days[5].




