Anaplastic Lymphoma Kinase (ALK)
Introduction
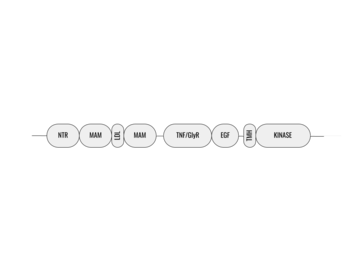
Figure 1. Outline of the domains and regions of anaplastic lymphoma kinase
Anaplastic lymphoma kinase is a receptor tyrosine kinase (RTK) important to the regulation of functions within the central nervous system [1]. RTKs are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. ALK activates pathways that promote cell growth and proliferation, similar to the Insulin Receptor (IR). These pathways include, but are not limited to, the ERK, JAK, and PI3K pathways. ALK is composed of two identical monomers consisting of seven unique domains and two intermixed regions. One region to note is the glycine-rich region which is highly uncharacteristic in that it has helices composed of only glycine. The preferred ligand for binding is AUG which binds in a dimeric fashion to ALK. When the ligand has bound, there is a conformational change that is covered in more detail in Figure 2 and it's accompanying text. In order to determine the atomic details of human ALK dimerization and activation by AUG, the methods of cryo-electron microscopy,nuclear magnetic resonance, and X-ray crystallography were utilized [1] . Anaplastic lymphoma kinase is a proto-oncogene with mutations associated with various types of cancers, including non-small-cell lung cancer, anaplastic large cell lymphoma, squamous cell carcinoma, and inflammatory myofibroblastic cancer [2]. ALK is a referred to as a proto-oncogene because certain mutations in it's protein sequence are known to have a strong positive association with the development of cancerous cells.
General Structure
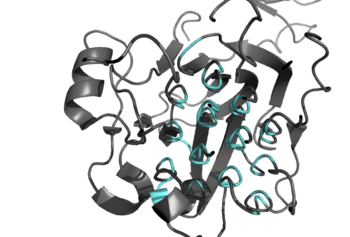
Figure 2: Shown in teal is the Glycine Rich Region of ALK in its helical structure.
Anaplastic lymphoma kinase is a , each consisting of seven domains and two regions [3]. These domains and regions are as follows: N-terminal region (NTR), two meprin–A-5 protein–receptor protein tyrosine phosphatase μ domains (MAM), low density lipoprotein receptor class A domain (LDL), (TNF), (GlyR), (EGF), transmembrane α-helix (TMH), kinase domain [1]. The NTR functions as a signal peptide, the structure of which is yet to be determined. Though the biological roles and structures of MAM and LDL have not been determined, they are a very unique component to ALK. ALK is the only RTK that has two MAM domains and a LDL domain. Studies of other MAM domains have suggested that MAM may play a role in cell-cell interactions through homophilic binding [4]. The TNF-like domain assists in mediating mature T-cell receptor induced apoptosis. Both the TNF domain and GlyR region are discontinuous, traversing each other frequently [1]. The GlyR region consists of multiple glycine helices which is a highly unique structure. Though the function of ALK's EGF domain is unknown, we do know that all EGF domains are found in the extracellular region and are thought to be important building blocks for extracellular proteins [5]. The TMH connects the extracellular and intracellular regions of ALK through the plasma membrane. The kinase domain is in the intracellular region and is phosphorylated at positions through the tyrosine phosphorylation mechanism in order to begin signaling cascades [5]. The structures of the N-terminal region, MAM, and LDL have not been determined. Only the TNF, GlyR, and EGF portions of ALK are required for ligand binding. All portions of anaplastic lymphoma kinase are located in the extracellular domain except for the transmembrane α-helix which is in the transmembrane region and the kinase domain that is located in the intracellular region.
Ligand Binding
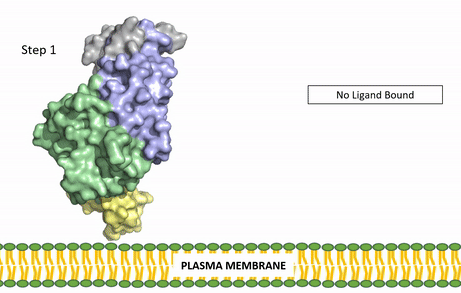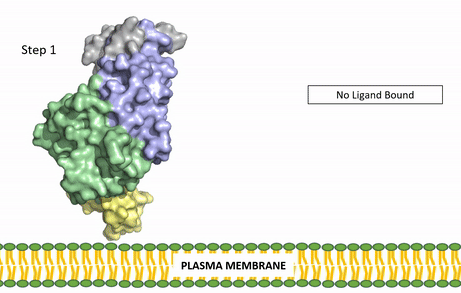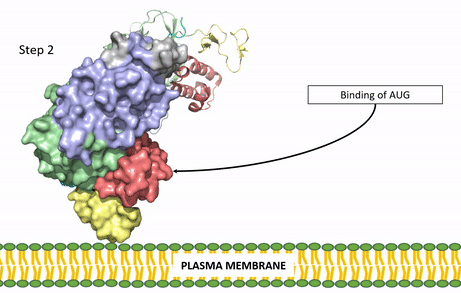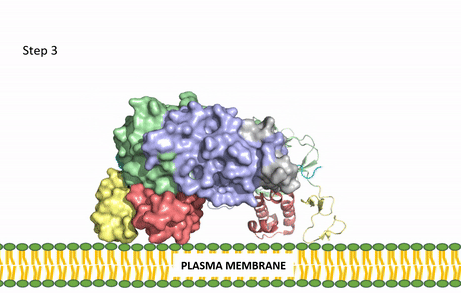
Figure 3: Gif-image of the conformational change occurring in the extracellular region of Anaplastic Lymphoma Kinase once the AUG ligand has bound to the ligand-binding site. This change is stabilized through contacts of the AUG and the plasma membrane. The video was made using stop motion animation techniques, then converted to gif format using EZgif.
The ligands recognized by anaplastic lymphoma kinase are FAM150 in a monomeric fashion and in a dimeric fashion [6] . Its biologically preferred ligand is AUG, a 128 monomer peptide ligand. FAM150 is a structural ortholog of AUG. The binding of ALK to it's ligand results in homodimerization and a conformational change. Prior to the ligand binding to anaplastic lymphoma kinase, the extracellular domain is oriented vertically and perpendicularly to the plasma membrane (Step 1, Figure 3). Once the ligand is (Step 2, Figure 3), ALK undergoes a conformational change and folds over so that the positively charged residues on the portion of the protein previously oriented vertically is now interacting with the negatively charged residues on the plasma membrane (Step 3, Figure 3). The residues of ALK and it's ligand interact through the formation of [7]. It has been hypothesized that the GlyR region plays a role in the flexibility needed to complete the conformational change. This conformational change via ligand binding induces the auto-activation of the kinase domain, in which the domains use the tyrosine phosphorylation mechanism to phosphorylate tyrosine residues on the opposite monomer. Another well-known RTK is the insulin receptor which is activated using a similar pathway. To learn more about the larger mechanism of RTK activation, click here.
Tyrosine Phosphorylation Mechanism
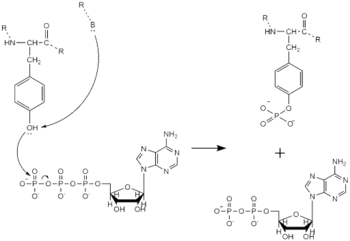
Figure 4. Tyrosine phosphorylation mechanism
Dimerization of anaplastic lymphoma kinase activates the of each monomer [6]. Next, the kinase domains phosphorylate the tyrosine residues (Y1278, Y1282, and Y1283 [8]) of the opposite monomer using ATP [7]. These phosphorylated tyrosine residues recruit signal proteins through phosphorylation. These signal proteins begin a signaling cascade by utilizing various signal pathways including the ERK, JAK, and PI3K pathways. These pathways signal for cell proliferation and survival (ex: begin transcription). The mechanism of tyrosine phosphorylation is a key step in signal transduction and regulation of enzymatic activity. This mechanism is widely used with a variety of proteins and enzymes including being used in insulin signaling. If there is a problem with this mechanism, it can lead to many ill effects as the signaling pathways instigated by this mechanism will be unable to function correctly.
Applications
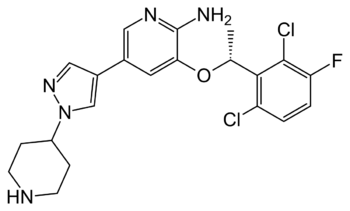
Figure 5. Structure of the ALK inhibitor, Crizotinib
Anaplastic lymphoma kinase's involvement as a proto-oncogene in various types of cancers has made it a target for drug therapies for these types of cancers [9]. These treatments are utilized on the basis that overactivation of the kinase domain of ALK by ATP binding is causing the cells to send out growth signals more rapidly than usual. This overexpression of growth signals allows the cancer cells to grow more rapidly than our normal cells. One therapeutic medication that has been increasingly used with high levels of success is . This medication was approved by the FDA in January of 2021 for the treatment of pediatric/young adult ALK-positive anaplastic large cell lymphoma. ALK-positive cancers are those in which the individual has an oncogenic mutation in their ALK protein sequence that is contributing to the proliferation of the cancer cells. The treatment has an overall response rate of 90%. Crizotinib works by to the ATP binding site of the kinase domain [10]. Crizotinib binds preferentially to ATP in this region and therefore can effectively block the binding of ATP [10]. With the binding of ATP being blocked, the intercellular signaling cascade cannot begin which works to prevent cancerous cells from growing and spreading . Crizotinib also potently inhibits the RTK, cMET, due to it's kinase domain ATP binding site being very similar to ALK's both sequentially and structurally [11]. Other than inhibiting cMET, critotinib is regarded as a highly successful drug as it is very specific to ALK's kinase domain ATP binding site [11].





