GLUT1
From Proteopedia
Facilitated Glucose Transporter 1, Solute Carrier Family 2, Homo sapiens
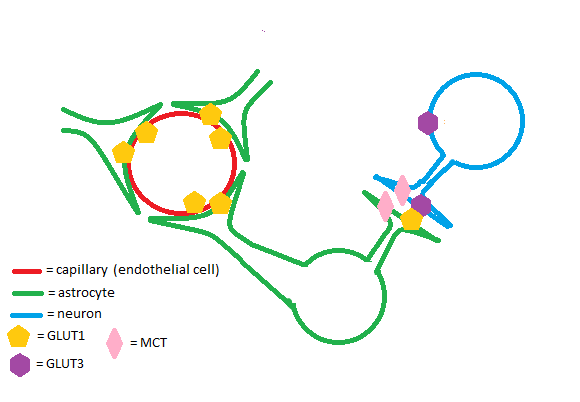
ClassificationGLUT proteins, encoded by the SLC2 genes, are part of the Major Facilitator Superfamily (MFS) of substrate transporters. Structural elements characteristic of GLUT proteins are twelve transmembrane domains, one N-linked glycosylation site, and lengths of about five-hundred amino acids.[1] GLUT proteins transport a variety of monosaccharides that enable cellular respiration and are thus abundant in the body. According to the CATH[1] classification database, the MFS superfamily has 23,982 unique species and 134 domains. FunctionThe GLUT1 is an insulin-independent glucose transporter expressed by all cells in the body to maintain adequate baseline glucose uptake. Some examples of tissues that express GLUT1 at high rates include: the placenta and fetal tissues, epithelial cells of the retina and mammary gland, and the brain.[2] BrainThe GLUT1 transporter works synergistically with other solute carriers in the brain. Astrocytes and endothelial cells of brain capillaries primarily express GLUT1; neurons primarily express Glut3. GLUT1 has a relatively high Km and is upregulated in times of hypoglycemia in the brain to ensure adequate glucose uptake. Glut3 has a low Km to ensure a steady supply of glucose for neurons even when extracellular glucose concentrations are low.[3] PregnancyThe GLUT1 transporter is highly expressed in both the placenta and the developing embryo/fetus.[4] During pregnancy, there is no significant fetal gluconeogenesis in humans.[5] High GLUT1 expression in the fetoplacental tissues ensures that the fetus receives a steady supply of glucose. RetinaGLUT1 is the most abundant glucose transporter in the retina. Retinal pigmented epithelium and retinal capillary endothelium, the tissues that comprise the retina-brain barrier, express GLUT1.[6] The retina has a consistently high energy requirement due to abundance of energy-consuming enzymes such as Sodium-Potassium ATPase. Studies on mice have shown that GLUT1 knockout leads to photoreceptor cell death - a greater effect was noted in rod cells versus cone cells.[7] DiseaseCancerOncogenes such as Ras and Src have been linked to upregulation of GLUT1 in rat tumors. Many studies have shown that GLUT1 is upregulated in malignancies of the breast.[8] Upregulation of the GLUT1 transporter has been shown to increase cell proliferation in some types of breast cancer.[9] RelevanceStructural highlightsThe GLUT1 transporter has one known site at Asn 45 and varied molecular weights of the GLUT1 transporter suggest glycosylation is dependent on cell type. This glycosylation site is thought to be important for glucose binding to the extracellular portion of the transporter. Mutations in the GLUT1 transporter from Asn 45 to an Asp, Tyr, or Gln residue have been shown to increase the Km of the enzyme.[10] has a and is proposed to be comprised of (view 2). References
| ||||||||||||