From Proteopedia
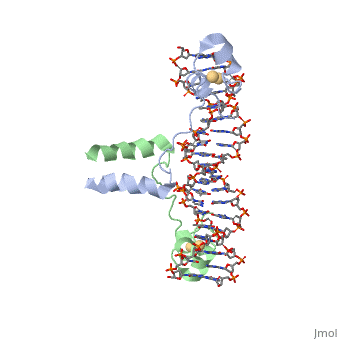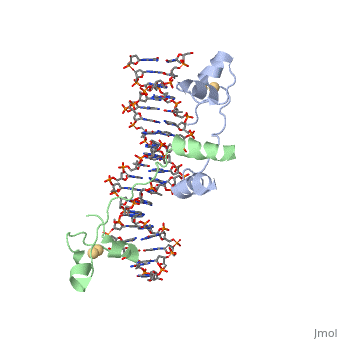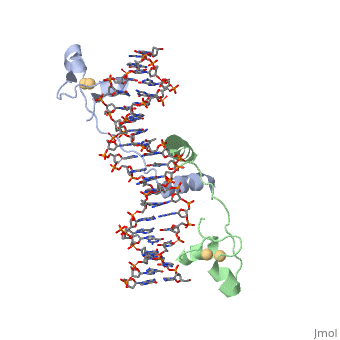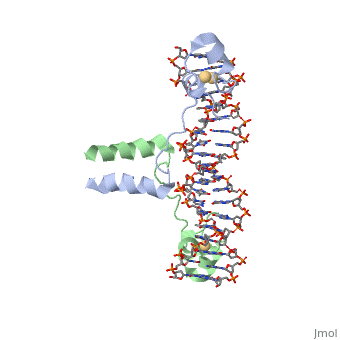
proteopedia linkproteopedia link==DNA RECOGNITION BY GAL4: STRUCTURE OF A PROTEIN/DNA COMPLEX==
| Structural highlights
Function
[GAL4_YEAST] This protein is a positive regulator for the gene expression of the galactose-induced genes such as GAL1, GAL2, GAL7, GAL10, and MEL1 which code for the enzymes used to convert galactose to glucose. It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes.
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
A specific DNA complex of the 65-residue, N-terminal fragment of the yeast transcriptional activator, GAL4, has been analysed at 2.7 A resolution by X-ray crystallography. The protein binds as a dimer to a symmetrical 17-base-pair sequence. A small, Zn(2+)-containing domain recognizes a conserved CCG triplet at each end of the site through direct contacts with the major groove. A short coiled-coil dimerization element imposes 2-fold symmetry. A segment of extended polypeptide chain links the metal-binding module to the dimerization element and specifies the length of the site. The relatively open structure of the complex would allow another protein to bind coordinately with GAL4.
: Zinc is responsible for maintaining the secondary structure.
Gal4 has lots of that interact with DNA
The Upstream activation sequence () for Gal 4.It only interacts with one strand of DNA sequence. The UAS is a 881 amino acid protein that binds to DNA, has dimerization, and recruits other transcriptional promoters. This needs galactose in order to be open.
It recognizes a 17 base pair sequence in (5'-CGGRNNRCYNYNCNCCG-3') the upstream activating sequence (UAS-G) of these genes. (This is what I found from sources)
i. I was searching for this, but I kind of struggled to find this when I was looking through the links.
ii. The protein interacts with one strand of double stranded DNA.
DNA recognition by GAL4: structure of a protein-DNA complex.,Marmorstein R, Carey M, Ptashne M, Harrison SC Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Marmorstein R, Carey M, Ptashne M, Harrison SC. DNA recognition by GAL4: structure of a protein-DNA complex. Nature. 1992 Apr 2;356(6368):408-14. PMID:1557122 doi:http://dx.doi.org/10.1038/356408a0
|
UvrD
, also known as Helicase II, is one of many components responsible in repairing DNA damage. Helicases use energy from ATP to unwind double helices in metabolic pathways using nucleic acids. ATP molecules are typically used to store energy shared between phosphate groups that gets released when breaking bonds to drive catabolic reactions.
Helicases were found in the 1970’s to be DNA-dependent ATPases, meaning that they use ATP hydrolysis to complete its interactions with the different types of nucleic acids it comes into contact with. Helicase II, also called UvrD is the founding member of SF1, one group of six superfamiliies used to identify helicases. SF1 and SF2 members share seven conserved sequence motifs that are involved in ATP Binding [1]. UvrD is important in replication, recombination, and repair from ultraviolet damage and mismatched base pairs. Nucleotide excision repair in a normal cell is supposed to correct pyrimidine dimers and other DNA lesions when bases are displaced from their normal positions. UvrD pairs up with the UvrABC endonuclease system, which works to displace the DNA. This is then repaired by PolI and DNA ligase [3]. A sub pathway of nucleotide excision repair is transcription-coupled repair, which works with an RNA polymerase to make repairs to damage to DNA [2].
UvrD Motifs
There are 7 sequence motifs and a Q motif conserved among the SF1 and the SF2. There are 4 domains that these motifs fit into (not shown). The domains are 1A, 1B, 2A, and 2B.I, Ia, II-VI are involved in ATP binding. Motifs Ia, III, and V are involved in ssDNA binding. Motif IV is reported to be unique in SF1. They found in their paper, seven new sequence motifs conserved among UvrD homologs. They are Ib, Ic, Id, IVb, IVc, Va, and VIa. These conserved residues are involved in DNA binding or domain 1B and 2B interactions [1].
In total, there are for UvrD, which are conserved in other homologous structures, such as PcrA, Rep, and Srs2. The homologous structures mentioned are Helicase 2 homologs, which appear in different species. These conserved motifs are important to maintain the function of[2]