Introduction
Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is a membrane transporter protein that is found in the plasma membrane of liver cells, or hepatocytes. NTCP's primary function is the transportation of taurocholates, or bile salts, into the liver and out of the liver to the small intestine [1] NTCP is part of the solute carrier superfamily, more specifically SLC10. NTCP is the founding member of the SLC10 family, first discovered in rat hepatocytes in 1978 [2] NTCP has a key role in Enterohepatic circulation, and it's unique ability to transport other solutes lends it therapeutic potential for lowering cholesterol and liver disease.
NTCP also serves as a binding site for hepatitis B virus and hepatitis d virus [3] Future studies into HBV binding mechanism can help understand infection pathways and the development of viral inhibitors.
Structure
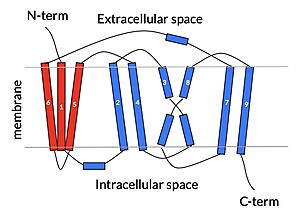
cartoon depiction of NTCP topology
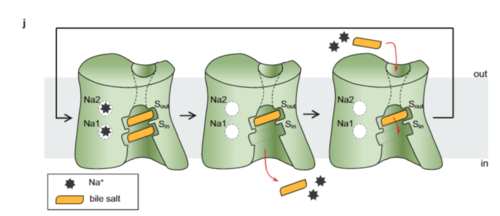
Sodium Taurocholate Co-Transporting Polypeptide, or NTCP, is one continuous polypeptide chain consisting of a total of 9 transmembrane α helices (TM1-9). There are two distinct domains within the quaternary structure of NTCP: a (blue)[4] It is understood that these sodium binding sites facilitate changes from open-pore to closed pore states of NTCP that allow for the binding or release of bile salts. Closed-pore state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. This also allows for sodium concentrations to regulate uptake of taurocholates. When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, closed-state is favored preventing diffusion of taurocholates. [5]
The is also characteristic of NTCP. The pore surface remains Hydrophobic, while lining of the open pore state is largely Polar. This pattern is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as steroids and statins. [5] Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant it is shown that some residues form Van der waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues.
Conformational Change
Bile Salt Transport
Medical Relevancy
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.