Sandbox Reserved 1780
From Proteopedia
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
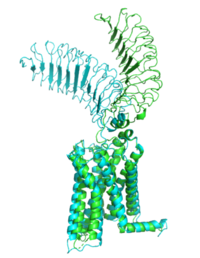
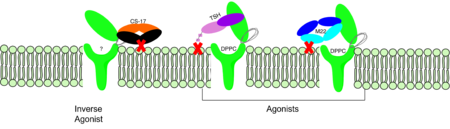
Active and Inactive FormThe TSHR protein exists in two states, active and inactive (Figure 1). The extracellular domain sticks out from the cell membrane into the space outside the cell. The contains 7 alpha helices that reside within the cell membrane. The exists when bound to the thyroid stimulating hormone (TSH) (GREEN LINK). One proposed mechanism for the transition from active to inactive describes that in its natural state, the TSHR ECD can spontaneously transition to the upstate, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of a clash with the cell membrane.[1] Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. [1] TSHR Agonists and Inverse AgonistsChemical agonists are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical inverse agonists bind to the same receptor as the agonist but promotes a biological response opposite that of the agonist. Different types of agonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead researchers to uncover the receptor's binding mechanism. M22 AgonistM22 is a monoclonal antibody that was isolated from a patient with Graves' Disease. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. [2]. The M22 autoantibody activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. [1] Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.[1] This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. CS-17 Inverse Agonist==Introduction== The Thyroid-stimulating hormone receptor (TSHR) is a G protein-coupled receptor (GPCR) that plays a crucial role in regulating the function of the thyroid gland. TSHR is a transmembrane receptor located on the surface of thyroid follicular cells, which are responsible for producing thyroid hormones. TSHR is activated by Thyroid-stimulating hormone (TSH) and triggers a signaling cascade that results in the production and secretion of thyroid hormones. Dysregulation of TSHR can lead to a variety of thyroid disorders, including hyperthyroidism and hypothyroidism. ==Structure== The TSHR protein is a large glycoprotein consisting of 764 amino acids. It is composed of three main domains: the extracellular domain, the transmembrane domain, and the intracellular domain. The extracellular domain of TSHR contains a leucine-rich repeat (LRR) domain, which is responsible for binding to TSH. The transmembrane domain is composed of seven alpha-helices, which span the lipid bilayer of the cell membrane. The intracellular domain is responsible for activating downstream signaling cascades upon TSH binding. [[File:PDB 1hyt.png|thumb|TSHR protein structure from PDB 1HYT showing the extracellular, transmembrane, and intracellular domains. Click on specific regions to see more details. Scene Authoring Tools can be used to create hyperlinks.]] ==Agonists== Several TSHR agonists have been identified, including M22 and CS-17. M22 is a monoclonal antibody that binds to the extracellular domain of TSHR and mimics the action of TSH, leading to increased production and secretion of thyroid hormones. CS-17 is a small molecule that also binds to the extracellular domain of TSHR and activates downstream signaling pathways. ==Ligands== Recent studies have identified other compounds that can bind to TSHR and modulate its activity. ML109 is a small molecule that can interact with TSHR and modulate its activity, but the precise mechanism of action is still unknown. True DPPC, a phospholipid found in lung surfactant, has also been shown to bind to TSHR and regulate its activity. However, the physiological relevance of this interaction is still unclear. ==Function== The TSHR protein plays a critical role in regulating the production and secretion of thyroid hormones. When TSH binds to TSHR on the surface of thyroid follicular cells, it activates a signaling cascade that leads to the production and secretion of thyroid hormones. This process is regulated by a negative feedback loop, where increased levels of thyroid hormones in the bloodstream inhibit the production and secretion of TSH from the pituitary gland. ==Clinical significance== Dysregulation of TSHR can lead to a variety of thyroid disorders. Hyperthyroidism, a condition characterized by an overactive thyroid gland, can be caused by the overstimulation of TSHR by TSH or TSHR agonists. This can result in an excess of thyroid hormones in the bloodstream, leading to symptoms such as weight loss, rapid heartbeat, and tremors. Hypothyroidism, a condition characterized by an underactive thyroid gland, can be caused by the decreased stimulation of TSHR by TSH. This can result in a deficiency of thyroid hormones in the bloodstream, leading to symptoms such as fatigue, weight gain, and cold intolerance. In addition, mutations in the TSHR gene can lead to inherited thyroid disorders. ==References==
==External links== *TSH Receptor on Proteopedia. | ||||||||||||