This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
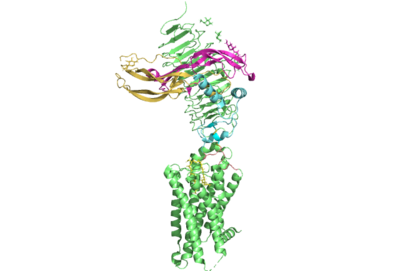
Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, the P10 peptide in pink, and thyrotropin bound in pink and yellow.
Thyroid hormones exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones is the cause of many disorders related to hypo- or hyperthyroidism. Thus, understanding the signaling of synthesis and release of these hormones is essential to the development therapeutic drugs to combat specific thyroid hormone disorders[3]. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone, commonly referred to as TSH or thyrotropin. The thyrotropin receptor is a G-protein coupled receptor that binds TSH and transduces signal to initiate synthesis and release of thyroid hormones. It is important to note that autoantibodies may also bind to this receptor causing inhibition or activation of its desired function. (Figure 1)[4][5]
Structure
Overview
The thyrotropin receptor has an extracellular domain (ECD) that is composed of a as well as a hinge region. This links the ECD to the seven transmembrane helices , which span from the extracellular domain to the intracellular domain [6]. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective when in the active and inactive states.
Hinge Region and P10 Peptide
Several parts of TSHR are very important for the functioning of TSH signaling. The is a scaffold for the attachment of the ECD to the 7TMD. Also, this region, has been found to have impact on the binding potency of TSH as well as intracellular cyclic adenosine monophosphate (cAMP) levels, which are partially mediated by the activation of the GPCR. Several features of this region have been found to be crucial to the potent activation of of the TSHR by TSH.[7]. The most important feature of the hinge region is the interaction of the with the through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1)[8]. These disulfides are critical as they link the ECD, where thyrotropin binds, to the TMD, whose conformational changes directly mediate the activation of the receptor's complementary G-protein. Movement of the ECD, caused by TSH binding, will cause rotation of the hinge helix and subsequent movement of the p10 peptide leading to movement of the transmembrane helices which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing Y385 and D386. Y385 is buried into a hydrophobic region of TSH while D386 forms a salt bride with R386 of the hormone. Together, assist in facilitating the stable binding of TSH to the TSHR [4]. This being said, it is important to acknowledge, the hinge region itself is not required for the activation of the receptor. In many of the aforementioned misregulations of thyroid hormone release, auto-antibodies are responsible. These auto-antibodies bind to the receptor through alternative interactions which cause conformational changes to the 7TMD without any need for a conformational change or extensive interactions with the hinge region[8].
7 Transmembrane Helices
The thyrotropin receptor is anchored to the membrane through seven transmembrane helices which is characteristic of GPCRs. Conformational changes in this region of the receptor are responsible for the activation of associated G protein[6]. In thyrotropin binding, changes to this region are mediated by movements in the afforementioned p10 peptide. When the hinge helix rotates, it causes p10 peptide displacement that allows the of the transmembrane domain to migrate towards the center of the 7TMD to increase van der waals contacts. In addition, lysine 660 of TM7 forms an with glutamate 409 of the p10 region. This interaction assists in the stabilization of the active state of 7TMD. In addition, the movement of the hinge helix has been found to bee associated with movement of a tyrosine residue relative to and isoleucine residue on the neighboring extracellular loop 1 (ECL1) helix. The identity of these residues has been found to be an important predictor in the activation of the thyrotropin receptor. For instance, structurally guided mutagenic studies have shown that the mutation of the isoleucine to a more sizeable phenylalanine decreases TSH signaling potency[8][9].In addition to these conformational changes, the sixth transmembrane helix of TSHR is also moved outward from the center of the 7TMD to facilitate alpha helix 5 of the alpha subunit of the G protein, which is the domain that becomes activated[8][10].
Relevance
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.

