Introduction

Schematic representation of SHOC2-PP1C-MRAS pathway after all domains are bound together.
SHOC2-PP1C-MRAS is a human enzyme that is involved in regulating cell proliferation and division [1]. The enzyme is involved in the vast RAS-MAPK pathway, which is initially activated by an extracellular growth factor binding to a membrane bound RAS GTPase such as HRAS, NRAS, or KRAS. RAS-GTPases are a family of proteins that work by functioning as molecular switches. This occurs from the protein alternating between binding GTP to be active and GDP to be inactive [1]. After activation via an extracellular growth factor, the RAS-GTPase enzyme binds GTP, which activates RAF[2] by phosphorylating the serine 259 residue. RAF triggers a series of downstream signaling pathways including MEK and ERK. The Ras/Raf/MEK/ERK pathway is a critical signaling cascade for activating transcription factors and regulating gene expression[3].
Structure
Overview
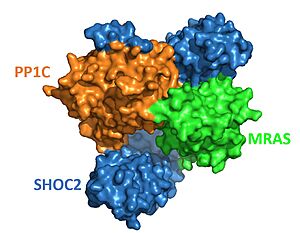
Figure 1. Surface representation of SHOC2-PP1C-MRAS from PDB 7pui. Blue is SHOC2, orange is PP1C and green is MRAS.
The enzyme requires 3 domains, SHOC2(blue), PP1C(orange), and MRAS(green), to form the active enzyme (SMP complex), also known as a holoenzyme (Figure 1)[4]. The SMP complex was determined via cryo-electron microscopy as well as x-ray diffraction. These studies found that PP1C and MRAS occupy the concave surface of SHOC2, leaving the catalytic site of PP1C and the substrate binding cleft in MRAS exposed.
SHOC2
SHOC2 is a scaffolding protein which acts as a cradle to bind PP1C and MRAS, allowing for the holoenzyme to be functional. The is a leucine rich repeat (LRR) protein that consists of 20 consecutive . This motif results in an extended beta sheet on the inner concavity of the protein surface with alpha helices facing outward. This results in a largely hydrophobic core[5].
PP1C
is a catalytic domain of a phosphatase enzyme PP1, which cleaves a phosphate. This subunit is a serine/threonine phosphatase known to be involved in a variety of cellular signaling pathways that control cell growth, division, and metabolism (again cite, see texts).
MRAS
is a GTPase protein and is anchored in the cell membrane. When MRAS binds GTP, it becomes active and triggers the assembly of the active holoenzyme[4]. MRAS is a close relative of the RAS protein and therefore shares most of it's regulatory and effector interactions[6]. A unique function of MRAS is it's ability to form a complex with SHOC2 and PP1, allowing it to have phosphatase activity.
Switch I and II
Switch I and II are located in MRAS. The switches determine whether MRAS can bind to SHOC2-PP1C. The switches have to go through a conformational change to allow binding of SHOC2-PP1C to MRAS. This conformational change is caused by GTP replacing GDP. Once GTP is added MRAS shifts and binds with SHOC2. When GDP is bound to MRAS, switch II is moved outward which causes a steric clash with SHOC2 . When GTP is bound, switch II can form various hydrophobic interactions with SHOC2[7] . Interactions are strengthened with hydrogen bonding and pi stacking. When MRAS is bound to SHOC2-PP1C, switch I has an important role in making interactions with PP1C.
Special Interactions
MRAS binds to SHOC2 exclusively through this concave region [8], primarily by the descending loop and strands of each LRR domains 2-10. This reaction is stabilized through between residues of each subunit. with the ascending loops of the SHOC2 LRR regions, and is further engaged through the N-terminal region containing the [9]. The initial forming of the complex begins with SHOC2-PP1C engagement, then is completed and stabilized by the GTP-loaded MRAS binding [9]. Once associated with SHOC2, and guides the holoenzyme complex to the cell membrane to begin signaling[8].
Mechanism
Once the SMP complex comes together, it plays a key role in regulating the activation of the RAF and MAPK pathway. To do so, PP1C, enhanced through the interactions with SHOC2 and MRAS, dephosphorylates a specific phosphoserine on RAF kinases. Doing so regulates cell growth, survival, proliferation, and differentiation [10].
Active Site
Once all the domains are bound NTpS binds to PP1C in the active site. Once NTpS is bound it becomes dephosphorylated and the complex falls apart. NTpS is dephosphorylated to prevent the active dimeric RAF from inactivating and changing into its monomeric structure. The is surrounded by hydrophobic and acidic regions along with the C-terminus. These regions are located on the surface of PP1C whereas the active site is placed further into the structure. It is thought that these regions help the ligand bind to the active site by making interactions that will lead NTpS into the protein. There is still some uncertainty as to how the substrate selectivity works but these regions could play an essential role in it. Specifically, the pS from NTpS would bind to the hydrophobic region on PP1C[11].
NTpS can bind to the PP1C active site without PP1C also being bound to SHOC2 and MRAS, however the catalytic activity is much slower and the reaction is less efficient. Also for this event to occur NTpS would need to be exposed from its binding site in the inactive RAF complex. A RAS has to bind to RAF to expose the NTpS allowing PP1C and NTpS to bind.
Implications
Because of this complex's key role in the regulation of the MAPK-RAF pathway, minor changes can be drastic. Unregulated activation of the MAPK pathway is known to be one of the most common causes of human cancer. This is due to unchecked cell division and proliferation which are the common trademarks of cancer. Cancer is thought to be so prevalent from this pathway because mutations that stabilize the interactions of the SMP complex consequently enhances holophosphatase activity [9], leading to accelerated cell division.
The unregulated MAPK pathway is also thought to be responsible for a multitude of developmental disorders commonly known as RASopathies [10]. One such RASopathy caused by the mutation of a RAF kinase is known as Noonan Syndrome (NS), which enhances complex formation. NS is a genetic disorder that can prevent normal development in different parts of the body in a variety of ways. Though specifics are related to the gene carrying the mutation, it can be characterized as having unusual facial characteristics, short stature, a variety of heart defects and disease, and other physical problems and developmental delays.


