Introduction
Significance and Background
History and Discovery
Structure
Transmembrane Region
The IgM BCR is tethered to B-cell membranes through the which is broken up into both extracellular and integral domains. IgM BCR assembly requires dimerization of the Ig alpha and Ig beta subunits which embed within the B-cell membrane. The is dimerized in both the integral region, with a hydrogen bond, and within the extracellular region with a . Although the mechanism of disulfide bridge formation is still unknown, it is believed that via N-linked glycosylation (NAGs, shown in green) of various asparagine residues in the extracellular region of both the alpha and Ig beta chains help to facilitate this process. Chaperon proteins remain bound to the alpha and beta subunits until both dimerizations occur at which point the rest of the BCR complex can be recruited.
After Ig alpha/Ig beta dimerization the transmembrane helices of the heavy chains can embed within the B-cell membrane as well. The side chains of this are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the phospholipid bilayer. A total of 9 polar residues (picture that zooms in here??) between each of the heavy chains are included on the interior of the helix structure which interact with a few polar residues on the Ig alpha and Ig beta chains to hold the complex together. Additional interactions between the Ig alpha/Ig beta dimer and the heavy chains occur in the constant region.
Fc Region
Fab Region
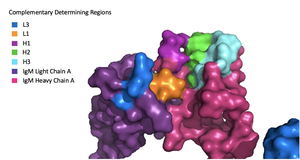
Figure 1. Surface Representation of IgM Antibody Binding Pocket
Signal Transduction
Relevance

