Sandbox Reserved 1774
From Proteopedia
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
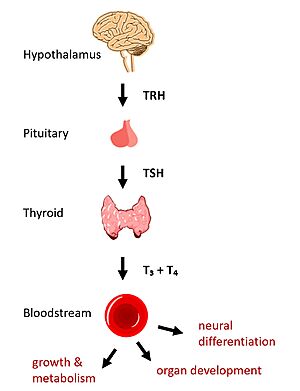
Thyroid Stimulating Hormone Receptor (TSHR) Structure and FunctionIntroductionIn humans, the hypothalamic-pituitary-thyroid (HPT) signaling axis regulates functions including metabolism, growth, organ development, and neural differentiation [1]. In this pathway, the thyroid stimulating hormone receptor (TSHR) activates transcription of thyroid hormones in response to ligand binding by thyroid stimulating hormone (TSH). After a brief introduction to the biological significance of TSHR, this page explores the structure of TSHR and its significance to TSH binding and receptor activation. Biological Significance of TSHRThe HPT signaling axis involves the brain, thyroid gland, and bloodstream circulation. In the first step of the pathway, thyrotropin releasing hormone (TRH) is secreted by the hypothalamus, which in turn stimulates the anterior pituitary gland to produce TSH [1]. TSH binds to TSHR on the surface of thyroid cells and triggers the production of thyroid hormones thyroxine (T3) and triiodothyronine (T4) through G-protein coupled receptor (GPCR) signaling [2]. T3 and T4 circulate in the bloodstream and enter cells via thyroid hormone transporters to regulate metabolic functions. Additionally, T3 and T4 act in a negative feedback loop to inhibit further TSH production [1]. Dysregulation of TSHR can lead to disease. In Grave's disease, antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism [2]. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated [1]. Structural Overview of TSHRThere are three main domains of the thyroid stimulating hormone receptor. First is the shown in green, which is concave in shape. It is also called the leucine rich region because it is made primarily of beta sheets which are rich in leucine [3]. This domain also contains lysine residues which play a key role in binding. Second is the shown in pink. This domain is made up of seven helices and undergoes a conformation change upon ligand binding that activates the GPCR signal cascade [4]. The third region of the TSHR is the shown in orange. The hinge region plays a key role in the movement and stability of the TSHR. TSHR ActivationHinge MotionCentral to the biological function of TSHR is its hinge motion which allows for transition between the . Deformation of the hinge region accommodates up-and-down rotation of the extracellular domain as a rigid body about an imaginary 55 degree axis. When the extracellular domain is upright, the receptor actively signals for thyroid hormone production. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. Notably, transition between the two states occurs spontaneously; favoring of the active or inactive conformation is influenced by hinge interactions and ligand binding [5]. Two observations help to explain how hinging of the extracellular domain can lead to signaling activation:
An animation of the hinge motion can be viewed here: Image:TSHR MorphBetterAngle.mp4. Stabilizing Interactions in the HingeTo understand stabilizing interactions which accommodate the hinge motion, the hinge region can be subdivided into the , which lies at the intersection of the extracellular and transmembrane domains; , which sticks up and serves as a binding platform for the TSH ligand; the region, which connects helix 1 with the p10 region; and the region, a conserved 10-amino acid sequence which connects to transmembrane helix 7 and undergoes most of the deformation [5] [4]. Two key disulfide bridges within the hinge region help to maintain its structure and orientation [4].
The upright, active conformation of the hinge is stabilized by its respective interactions with the EC and TM domains [5].
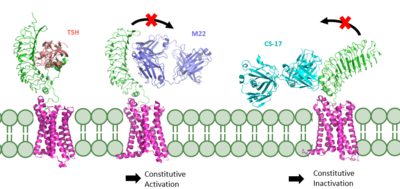
If the stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine strengthens the hydrophobic interaction too much, leading to overactivation. Contrastingly, TSHR underactivation results from disrupting the ionic interaction with an E409A mutation, which is associated with diminished receptor activation and TSH potency [5]. Ligand BindingBinding of Thyroid Stimulating Hormone to TSHRThe thyroid stimulating hormone by complementary shape. The ECD is curved and compliments the curvature of TSH similar to how a baseball fits into a glove. There are also several key ionic interactions between the TSH and TSHR. The key ionic interactions occur in the which is highlighted in yellow. The seatbelt region is located in the beta subunit of the TSH. is Glu118 from TSH and Lys58 from the ECD. is between Asp111 from the TSH and Lys209 from the ECD. These interactions form salt bridges between the ECD and the TSH which allows for specificity of binding for TSH to TSHR [4],[5]. Other key interactions that allow for specificity of binding are between TSH and helix 1. Helix 1 contains several polar residues that interact with surrounding nonpolar residues like Leu62 and Phe17. Positively charged Arg54 was also seen to interact with Helix 1. These interactions increase the activation potency and help activate the push and pull mechanism of the hinge region [4],[5]. Blocking TSHR in Active/Inactive StatesThe interactions between the ligand and the receptor have important consequences for disease states. In the image shown to the right are three different states of TSHR. The left-most structure is TSH bound to TSHR in the upright active conformation. In the middle receptor-ligand pair, M22 is bound to TSHR is in the upright state and prevents transition to the down state because of steric clash with the membrane. This conformation causes constitutive activation and the elevated levels of thyroid hormones which are found in a person with Grave's disease. On the right-most side is CS-17 bound to the TSHR. In contrast to TSH and M22 binding, CS-17 binds and locks TSHR in the down, inactive conformation. This prevents the signaling cascade to translation and causes constitutive inactivation [5]. These different ways to active and inactive TSHR could represent potential therapies for someone with Grave's disease or other thyroid-related diseases with overactive TSH binding. Whereas current therapies target T3/T4 synthesis or destroy the gland using artificial hormones, these diseases could instead be targeted with something like CS-17 which would compete with M22 and TSH to lessen overactivation. References
| ||||||||||||