Introduction
B-cells play an important role of the human immune system and can be found circulating throughout the body. On the surface of B-cells, membrane bound B-cell receptors(BCRs) can be found [1]. These complex proteins are made up of membrane bound immunoglobulins (mIg). There are several different types of BCRs, namely IgG, IgA, IgM, IgE, or IgD. Each specific BCR has important functions for different diseases, but the IgM BCR in particular is most interesting. The BCR consists mainly of three domains: extracellular, transmembrane, and intracellular. While the extracellular region makes up most of the protein, perhaps the most interesting interactions can be found in the transmembrane domain. Unlike other BCRs, the IgM BCR has a specific heavy chain interaction with the α-β subunit of the protein[2]. The role of BCRs is to bind to foreign antigens and initiate the appropriate immune response. Once bound to an antigen, the IgM BCR undergoes a conformational change in the extracellular region. While the exact conformational change is still not known, preliminary studies suggest that there is separation of Fab fragments that opens the binding site within the BCR. This initiates several signal transduction pathways, which are responsible for processing the antigen and initiating the appropriate immune responses. More specifically, the α-β subunit is connected to the phosphorylation of an immunoreceptor tyrosine-based activation motif(ITAM) upon binding. This in turn triggers the activation of kinases downstream that aid in the immune response.
Structure
B cell receptors have distinct functional domains each playing a unique role in response to a foreign antigen. These include the extracellular domain, the transmembrane domain, and the intracellular domain (the structure of this domain is still unclear). To accomplish B cell signaling, the BCR must bind an antigen and transmit the signal through the receptor domains. Exploring the different sections of a BCR, the antigen binding site (located in the extracellular region) is specific to antigens, but the process is highly conserved across different BCRs. More specifically, the IgM BCR has a unique interaction concerning its Fc chains and a/b subunits. These interactions contribute to the overall structure of the protein. This section will explore these structural regions starting with the extracellular domain, describing the antigen binding domain, outlining unique interactions between Fc chains and a/b subunits, and describing the intermolecular interactions that keep subunits together. Ultimately, the interactions of the transmembrane domain that anchor the complex to the membrane are examined.
Antigen Binding Site
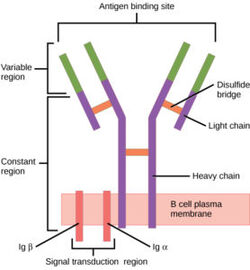
Figure 1. Overview of the human B Cell Receptor and its structural components. Used with permission under Wikimedia Commons.
The binding of an antigen to the human B Cell receptor is identical to other common soluble antibodies (such as IgG, IgA, IgM, IgE, or IgD). The antibody portion of the B Cell Receptor is roughly "Y" shaped and consists of two identical and two identical chains creating two similar epitope binding regions[3] (figure 1). Two antigen molecules can bind independent of one another to produce a response. Matching with standard FABs, the Ig portion has constant and variable region. The stem of the "Y" is a (Fc) composed of only heavy chain interactions[3]. The two heavy chains then branch at a flexible . These interact individually with one light chain creating two Fab fragments or branches of the "Y"[3]. Light and heavy chains are held together via weak intermolecular forces and disulfide bridges[3]. Each then terminates with two (Fv). These variable regions consist of hyper-variable loops, desired random coils of amino acids selected for specific recognition of a desired antigen [3]. Binding to an antigen is determined based on intermolecular interactions to the hyper variable loops, and selectivity is provided by unique hyper variable loop sequences. Due to the identical structure of Fab fragments, BCR will recognize antigens in the same manner as do free antibodies. This is emphasized by Ma et al. who studied the IgG- BCR (VRC01) that targets gp120 of the HIV-1 virus. By comparing structures of membrane bound and free antibodies, the membrane bound BCR form has an identical structure to the free antibody suggesting that they recognize antigens in the same way. [4] This leads to a conformational change in the protein and transmits the signal through the membrane. [5]
Fc and α/β Interactions
While the antigen binding site structure of the mIgM BCR is identical to common soluble antibodies, intermolecular interactions between the heavy chains and Igα/β subunits provide the emergent receptor properties. In the Fc portion of the structure, the two heavy chains interact via a disulfide bond and form an . Additionally, the Fc portion binds the with 1:1 stoichiometry. [6]. Due to the orientation of the heavy chains in the O-shaped ring, only Heavy chain 1 (Hc1) forms direct interactions with the Igα/β heterodimer. To correspond with the 3D representations, Hc1 residues will be represented in blue, Igα residues will be represented in red, and Igβ residues will be represented in orange. Furthermore, through two hydrogen bonds (T75-Q487 and N73-Q493) which are stabilized by sandwiching of aromatic residues (W76 sandwiched between F358 and F485). Similarly, through three hydrogen bonds (Y66-R491, K62-T530, and R55-T533). The residues involved in the interactions at the heavy chain and Igα/β interface are highly conserved across all species, suggesting a conserved mode of interaction. [2]. The Igα/β heterodimer is composed of a transmembrane domain, which consists of two hydrophobic helices, and an extracellular domain, which forms an interface with and Hc1. The Igα/β heterodimer is an obligate component of all BCRs. Igα and Igβ non-covalently associate with mIgM, and are crucial components for initiating biochemical signaling inside the B cell upon antigen binding. [6]. by a disulfide bond between cystine residues (C119-C136). The disulfide bond is further stabilized by π-π stacking (Y122 and F52) and a hydrogen bond (G120-R51). These residues in Igα/β are highly conserved across species, suggesting conservation of the Igα/β interface. [2].
Transmembrane Interactions
Many transmembrane interactions can be found within a IgM B-cell receptor. The have numerous interactions that keep them associated with each other. An overview of all residue interactions can be found (highlighted in green). At a cellular pH, various amino acid residues found in the transmembrane region are charged as well, which strengthens the overall interaction. For example, between residues N155 and E138, along with numerous other hydrogen bonds, works to stabilize the α-β chain interactions. Further down the chains, between residues T166 and E148 also have strong hydrogen bonding. Overall, these hydrogen bonds and ion interactions work to maintain the association of the α-β chains.
Medical Relevancy
B-cell Formation
Figure 2. The Signal pathway for the response to an antigen by the B-cell receptor. Illustration reproduced courtesy of Cell Signaling Technology, Inc. (
www.cellsignal.com).
The formation of B-cells occurs in the bone marrow from hematopoietic stem cells[7]. Once formed, B-cell receptors are attached to B-cells through the aid of membrane-bound proteins in bone marrow cells. During this process, gene recombination occurs, which allows unique BCRs to become highly specific to different antigens. The complexity of the signal transduction pathway upon antigen binding is shown in figure 2.
Disease
B-cells and their respective receptors play an important role in the immune response. Misregulation can lead to damaging consequences. Autoimmune diseases develop when somatic cells are recognized as foreign antigens and the body tries to eliminate them [8]. B-cell receptors are hypothesized to be an essential part of autoimmune disease development due to BCR function and role in the immune systems. In autoimmune diseases, BCRs improperly recognize somatic cells from different tissues and elicit the production of(autoantibodies)[8], causing the destruction of these cell types. Examples of these diseases include rheumatoid arthritis where the lining of joints is targeted and degraded, multiple sclerosis which targets the myelin sheath that surrounds nerve cells, type 1 diabetes mellitus where the insulin producing cells are targeted for destruction, and systematic lupus erythematosus where multiple organ systems are targeted (skin, brain, lungs, and kidneys are common targets) [8].
Therapeutics
Current approaches to treatments of these autoimmune diseases include replacement and immunosuppressive therapies [9]. Replacement therapy consists of the supplementation of important biological hormones or molecules that are reduced from disease. Immunosuppressive therapies instead treat disease symptoms to prevent further organ damage [9]. Immunosuppressive therapies include drugs that suppress the immune system response as well as anti-inflammatory drugs. Gene therapy has also been studied as another therapeutic avenue. In gene therapy, cells express specific genes for the regulation of proinflammatory molecules or reduction of immune cells to the site of disease [10]. Currently, the majority of treatments for autoimmune diseases aim to improve the quality of life and reduce symptoms as there has not yet been an established cure.


