Phosphoenolpyruvate carboxylase
From Proteopedia
FunctionPhosphoenolpyruvate carboxylase (PEPC) catalyzes the irreversible β-carboxylation of PEP in the presence of HCO3- to yield OAA (oxaloacetate) and Pi ( inorganic phosphate), using Mg2 + as a cofactor [1]. It is a key element in the carbon fixation process in C4 plants [2]. 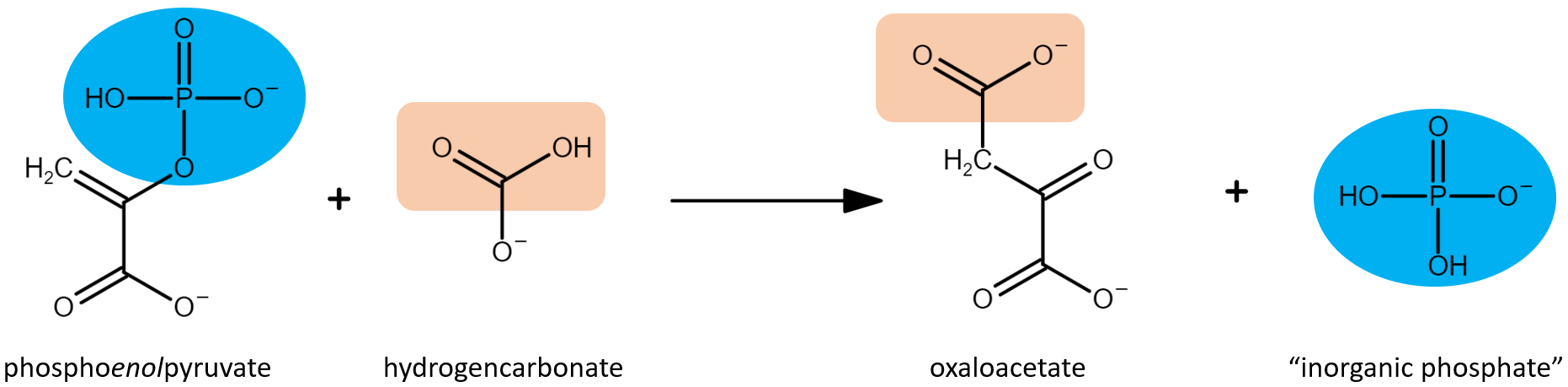 Figure 1 Schematic representation of PEPC catalysis of phosphoenolpyruvate carboxylation. Adapted from Svensson et al. 2003. [3]
OverviewThe enzyme phosphoenolpyruvate carboxylase (PEPC) catalyzes the carboxylation of phosphoenolpyruvate to form oxaloacetate, with Mg2+ or Mn2+ as essential cofactors [4][3]. It can be considered the key enzyme in the C4 photosynthesis process, once it’s a central part of the mechanism that makes C4 plants more efficient in carbon fixation compared to classical C3-photosynthetic pathway plants, especially in abiotic stress environments [5]. PEPC is a ubiquitous enzyme, present in the genome of all plants. However, the isoforms found in C4 metabolism plants differ in their kinetic and regulatory characteristics, when compared to C3 orthologs [6]. Among the Flaverina genus of the Asteraceae family, closely related C3 and C4 species are found, providing a good model to study the differences between the two processes. The comparative analysis of F. pringlei (C3) and T. trinervia (C4) PEPC’s, has shown that the exchange of single amino acids can be responsible for the observed differences in saturation kinetics and inhibitor tolerance between PEPC’s of C3 and C4 species [7][6]. PEPC and C4 photosynthesisIn the classical C3 photosynthetic pathway, the Rubisco enzyme, abundant in leaf mesophyll cells, is responsible for the carboxylation ribulose-1,5-bisphosphate (RuBP) and, therefore, for the assimilation of atmospheric CO2 into 3-phosphoglyceric acid (PGA). However, under high temperature or high concentrations of O2, Rubisco’s oxygenating activity is favored leading to the oxygenation of RuBP, which produces phosphoglycolate (PG) as well as PGA. While PGA can readily be recycled back to RuBP via the Calvin cycle, PG has to be first metabolized into pyruvate and then to PGA. This process is commonly referred to as photorespiration and it leads to significant losses in freshly assimilated carbon to the atmosphere, essentially diminishing photosynthetic efficiency [8][5]. Plants with C4 photosynthetic metabolism, on the other hand, show very low levels of photorespiration, and consequently are more efficient in terms of carbon fixation [8]. That is made possible by a complex reorganization of leaf anatomy and metabolism in a CO2-concentrating mechanism that inhibits the photorespiratory pathway [5][6]. Without this characteristics, efficient carbon fixation in the mesophyll and subsequent carbon concentration in the BS wouldn’t be possible. Therefore, the evolution of PEPC’s C4 isoforms is one of the most important steps in the establishment of the C4 pathway [2]. 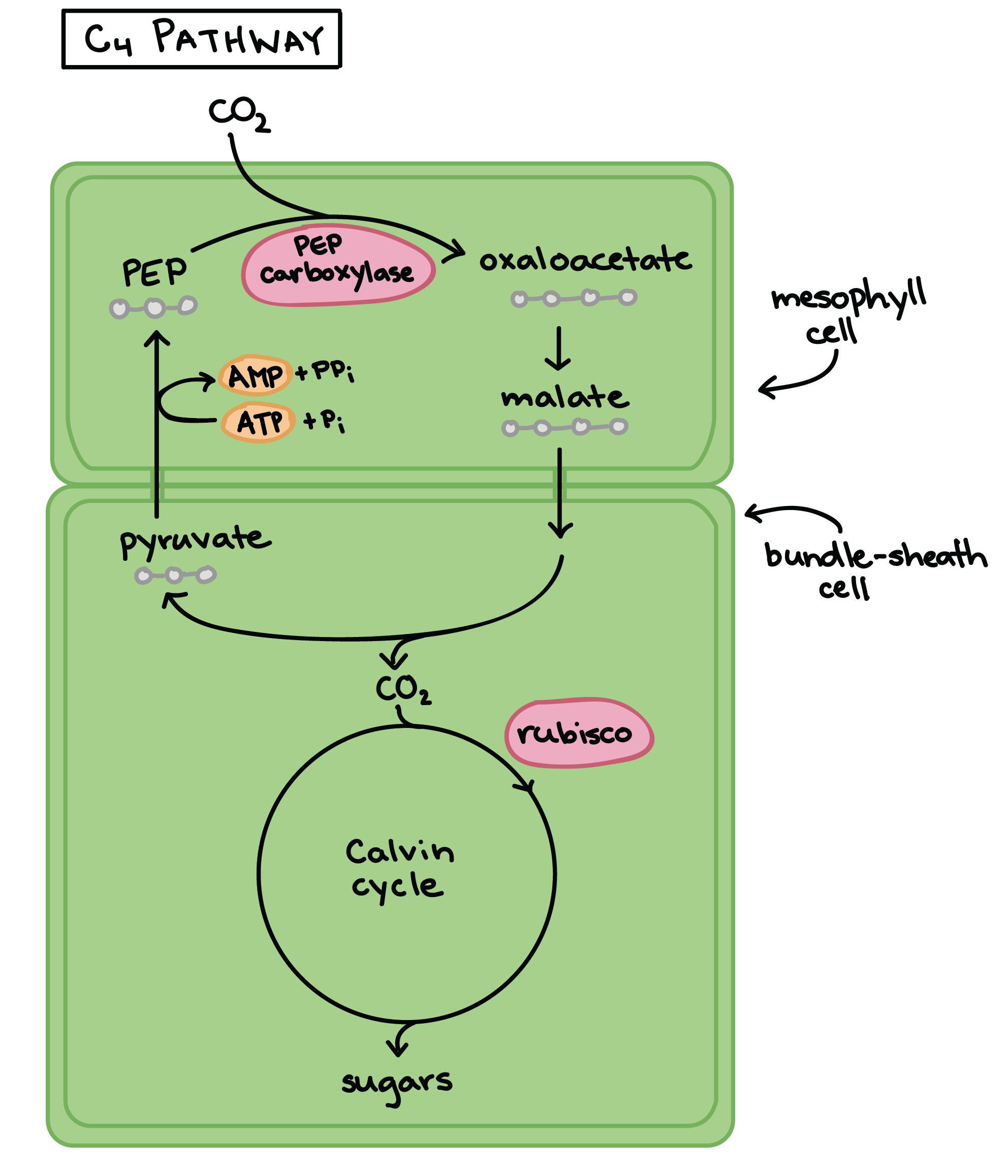 Figure 2 Diagrammatic representation of the C4 photosynthetic pathway as it occurs in plants of the NADP-malic enzyme (NADP-ME) subtype. Adapted from Sage et al. 2012. [5]
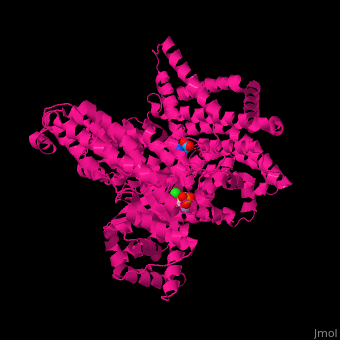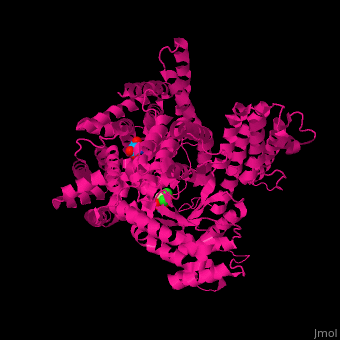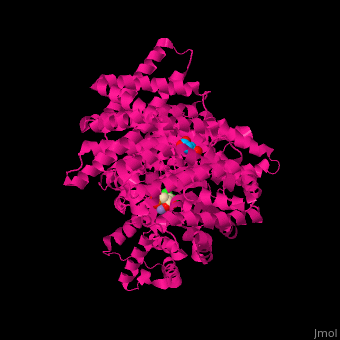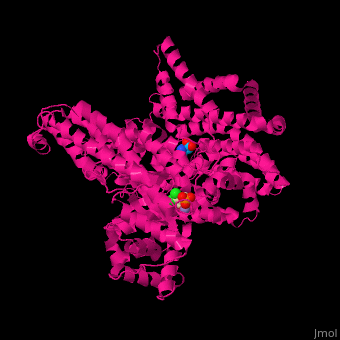
PEPC enzyme structureOverall, plant PEPC’s present a similar structure characterized by four 105-110kDa identical subunits with a conserved N-terminal serine-phosphorylation domain, forming homotetramers [4][1]. X-ray crystallography studies on Escherichia coli and maize (Zea mays) PEPC’s show that the tetramer is comprised of two pairs of monomers with a greater amount of intersubunit contacts, suggesting a homotetrameric structure of a ‘‘dimer-of-dimers’’. The dimers are held together through an interation between Arg-438 of one subunit and Glu-433 of the neighboring subunit, forming a salt bridge [9]. The monomeric structure maize’s C4-PEPC monomer consists of an eight-stranded β barrel and 42 α helices [4][10]. The of each monomer, bound to PEP and the cofactor, is located in the C-terminus region of the β barrel [11]. 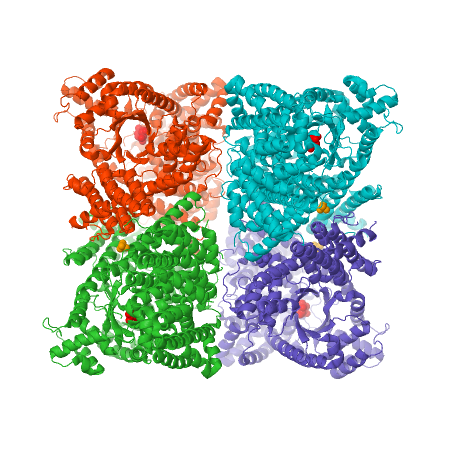 Figure 3 X-ray crystal structure of Flaverina trinervia’s C4 PEPC bound to glucose 6-phosphate (magenta). Two strongly bound dimers (left and right sides of the structure) form the tetrameric quaternary structure. Adapted from Schlieper et al. 2014. [12]
Allosteric regulation and reaction mechanismPEPC’s carboxylase activity is regulated by different post-translational mechanisms. In C4 and CAM plants, the phosphorylation of a serine residue near the N terminus (S15 in maize C4-PEPC) activates the enzyme by decreasing its sensitivity to allosteric inhibitors such as aspartate and malate and increasing activation by the positive allosteric regulator glucose 6-phosphate [1]. Studies on F. pringlei and T. trinervia, have positively identified the residues Arg641, Lys829, Arg888 and Asn964 as binding motif of the negative allosteric inhibitors aspartate and malate [6]. Similar studies have also identified the site in maize [11]. In C3 PEPC, Arg884 provides an additional hydrogen bond for inhibitor binding, whereas in C4 PEPC isoforms the substitution of this residue by a glycine, reducing the enzymes sensitivity towards both feedback inhibitors [13][6]. The positive allosteric effector glucose 6-phosphate’s binding site has also been identified in the C4-PEPC of maize. X-ray crystallography of maize’s C4-PEPC in complex with sulfate ion (a positive effector analog of glucose 6-phosphate) revealed that the positive effector was bound to the enzyme at the dimer interface and was surrounded by four positively charged residues (R183, R184, R231, and R372 in the adjacent subunit [4]. 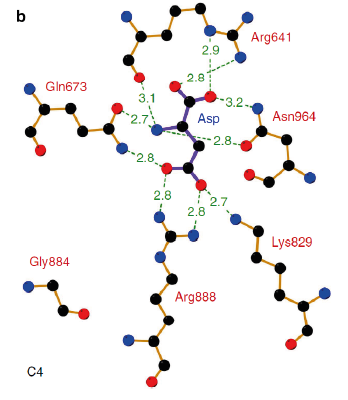 Figure 4 Inhibitor-binding site of Flaverina trinervia’s C4 PEPC. Adapted from Paulus et al. 2013. [6]
Most proposed catalytic reaction mechanisms suggest that PEPC catalyzes an ordered multistep reaction in which the preferred order of reactant binding to the active site are: first the bivalent cation ( Mg2+ or Mn2+), then PEP and lastly bicarbonate (HCO3- ) [4][10]. In a review article on the subject, Izui et al., 2004 [10], proposes a detailed reaction mechanism for the enzyme, based on maize and E.coli PEPC structures in which PEP is located in a hydrophobic pocket consisting of W248, L504, and M538 residues. In this model, H177 is a critically important catalytic base as it is supposed to play a role in stabilizing the carboxyphosphate intermediate and abstracting a proton from its carboxyl group. 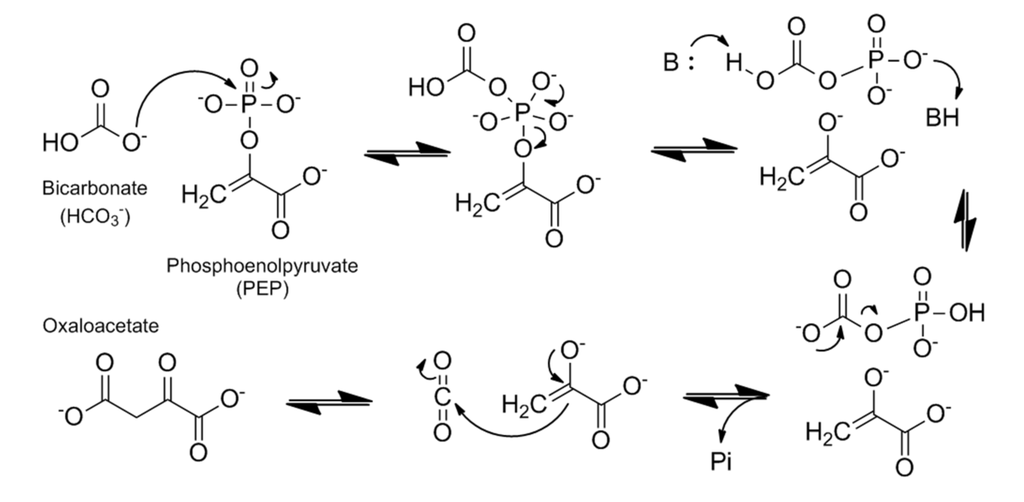 Figure 5 Catalytic mechanism of PEPC based on maize C4-PEPC and E. coli PEPC crystal structures and the three-step reaction model. The hydrophobic pocket is shown as yellow circles and the residues show maize numbering. Adapted from Izui et al. 2004. [10]
3D structures of phosphoenolpyruvate carboxylase1jqo, 6v3o – cPEPC – corn
Updated on 26-June-2023
References
| ||||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Lucas Xavier da Cunha, Michal Harel, Alexander Berchansky, Joel L. Sussman