User:Marcos Vinícius Caetano/Sandbox 1
From Proteopedia
Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure
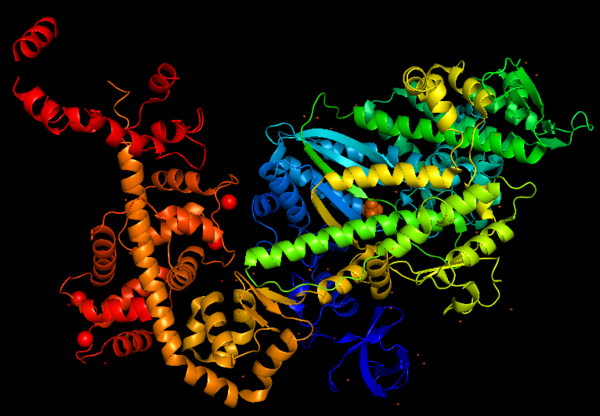
IntroductionMyosin consists of a superfamily of actin motor protein, being composed of at least 20 structurally and functionally distinct classes. Specifically in humans, there are 39 myosin genes, encoding 12 of these classes. Myosins use ATP hydrolysis to move molecular cargoes along the actin filaments inside the cell. To this day, all characterized myosins move toward the plus-end of the filaments, except for myosin VI, which moves in the opposite direction. Myosin VI is the only myosin that moves towards the minus-end of the actin filament and this unique property was the target of different studies throughout the years, and it is studied until nowadays, showing the versatility and importance of this protein. This is a figure of Myosin VI structure in gradient rainbow representation, where blue is N-terminal (5’) and red the C-terminal (3’). The basic structure of myosin VI, and most myosin molecule heavy chains, consists of three regions:
Obs.: The entry '2BKI' contains only the of myosin VI.
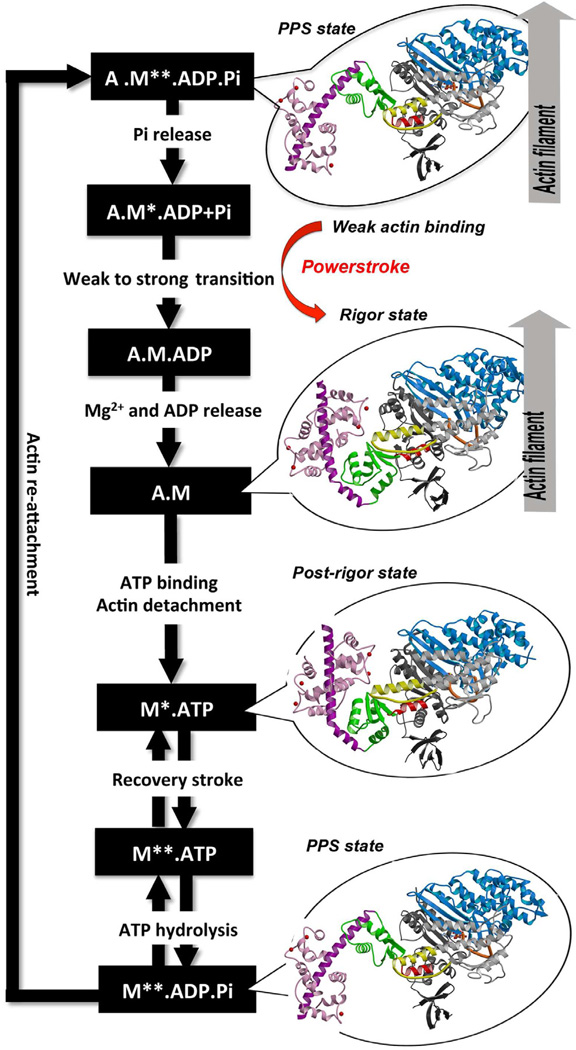
Predicted amino acid sequences and cDNA comparison of myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region. This is probably due to the unique and different functions of this protein. This scene represents the , where pink gradient shows the more conserved region, white is average, blue gradient is the variable region and grey or yellow is insufficient data. FunctionMyosins (including Myosin VI) are involved in a wide variety of functions, such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins. ATPase cycle of myosin VIStructure featuresThe crystal structure of the entry '2BKI' was determined by x-ray diffraction with the resolution of 2,90 Å and is composed by 3 chains: and two chains of (a protein that plays a major role in the Ca2+-dependent regulation of wide variety of cellular events).
Secondary structures The figure below portrays Myosin VI structure in terms of alpha helix (in red) and β-sheet (in yellow). The central β-sheet is the major component of the transducer and it can adopt differently twisted conformations depending on the nucleotide- and actin-binding states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi). This distortion also differs from classes of myosin.
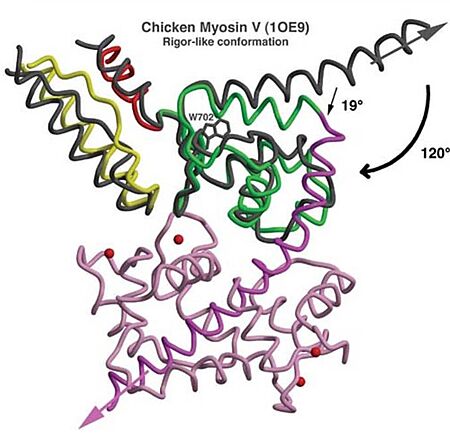
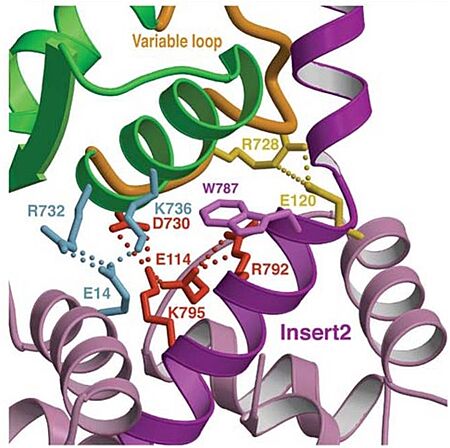
Unique features The unique function and distinct characteristic of myosin VI is due to its structure, having several unusual features, listed below.
Insert 1: .
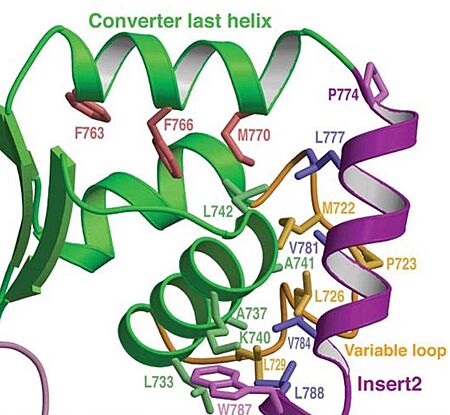
In addiction, there is apolar interactions that stabilize the proximal part of insert 2 on the surface of the converter. Hidydrophofobic side chains from the amino acids (F763, F766, M770) stabilize the orientation of the last helix. Also, there is salt-bridge interactions between the converter and both lobes of CaM. 3D structureAll 3D structure, models and scenes were based on 2BKI - Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure, published in https://doi.org/10.1038/nature03592 - Ménétrey, J., Bahloul, A., Wells, A. L., Yengo, C. M., Morris, C. A., Sweeney, H. L., & Houdusse, A. (2005). The structure of the myosin VI motor reveals the mechanism of directionality reversal. Nature, 435(7043), 779–785. References | ||||||||||||