Introduction

Figure 1. AMYR with RAMP3 and Amylin Peptide
The human amylin receptor is a G-coupled protein receptor.
Amylin
Amylin is a neuroendocrine hormone that is synthesized with insulin in the beta cells of pancreatic islets. It can cross the blood-brain barrier and regulates glucose homeostasis via inhibiting gastric emptying, inhibiting the release of glucagon, and inducing meal-ending satiety[1]. In doing so, it prevents spikes in blood glucose and overeating, making it a suitable target for Type 2 Diabetes treatments and therapies. Seeing as Type 2 Diabetes is a major risk factor for Alzheimer's Disease, as Type 2 Diabetes cases continue to increase, there will likely be a spike in Alzheimer’s Disease as well[2]. Therefore, it is vital that amylin, its receptor, and analogs, such as pramlintide, are understood to aid in rational drug design.
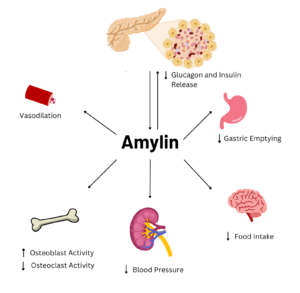
Figure 2. Effects of Amylin in Humans
Structure
Amylin Receptor
The amylin receptor (AMYR) is the result of the heterodimerization of the calcitonin receptor (CT) and a receptor-activity modifying protein (RAMP). The patterns of peptide interaction between CT and AMYR are very similar overall, but amylin has a higher affinity for AMYR1 and AMYR3 than AMYR2[3].
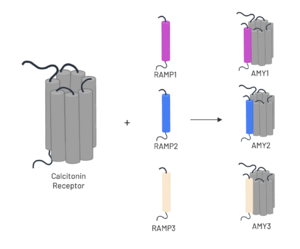
Figure 3. Heterodimerization of CT and RAMPs
Calcitonin Receptor and G-alpha Interactions
RAMPs
Amylin Binding
Amylin Receptor Functional Overview
Hydrophobic Interactions
on the C-terminus of the amylin peptide has extensive van der Waals interactions with the CT and RAMP3. The pi stacking and hydrophobic interactions increase the affinity and interactions between the amylin peptide, CT, and RAMP3, aiding in their association.
Amidated C-Terminus
R11
R18
T9 Water Network
Disease
Pramlintide Analogue
The first human amylin analogue, , was developed in 1995, and marked a significant advancement in the treatment of Type 2 Diabetes[4]. As of 2024, it is the only FDA-approved drug for the treatment of Type 2 Diabetes using the AMYR as a target. Recent studies in rodent Alzheimer’s Disease models suggest that pramlintide reduces amyloid-beta plaques, making it a potential therapeutic target for Alzheimer’s Disease[2].
There is limited knowledge about how human amylin binds to the human AMYR, so many studies utilize rat amylin as the peptide for the human AMYR. Rat amylin and pramlintide showcase very similar structures and maintain a high sequence similarity. For instance, the N-term lysine residue is conserved between rat amylin and pramlintide, as well as human amylin [3], suggesting that the lysine is integral for binding to AMYR. The residue changes between the two include , , L23 in rat amylin to F23 in pramlintide, and V26 in rat amylin to I26 in pramlintide. While these residues differ, the overall properties of the various residues remain consistent, so many of the same interactions with AMYR are likely still able to form.
Human amylin and pramlintide only differ at 3 residues, with all three being changed to proline in pramlintide. The mutations break the helical nature present in human amylin, which likely prevents the aggregation of amyloid beta plaques in Alzheimer’s Disease.



