Introduction
The Glucose-dependent Insulinotropic Polypeptide (GIP) is a ligand that can be bound to its receptor (GIP-R) to help facilitate the breakdown of glucose. This is the basis of what makes up one of the key ways that glucose is bound and broken down into different parts, including insulin, to be able to maintain blood glucose levels within the body. With this structure, it is also to work with the GLP-1 receptors found within cells to help break down glucose present within cells to create energy.
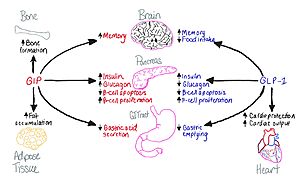
Figure 1: Disctinction of functions between the GIP and GLP-1 receptors.
Function
The GIP receptor helps facilitate movement of glucose within a cell. [1]. It has a natural ligand that is 42 residues and helps kickstart the GIP-R into firing, as a transporter for glucose in and out of the cell. Once the levels become too high, the ligand will send this transporter back down into the cell and not have anymore glucose activate. It will also cause the insulin pathway to start to help signal that there is too much glucose in the body.
Tirzepatide
Tirzepatide has been used as a treatment for Type 2 diabetes and It is used to help treat Type 2 Diabetes, as an agonist to allow insulin to be broken down. This also works in tandem with GLP-1... It also contains two residues with an AIB sequence, which stands for alpha-amino isobutric acid and aids with preventing degradation of the peptide [2].
Disease
Diabetes is a disease that causes
Structural highlights
Active Site
Main binding domains between tirzepatide and the GIP receptor would contain an arginine 190 and glutamine 220 residues to facilitate binding of the ligand. The binding is also able to use a tyrosine residue at position 1 that helps guide the ligand into the correct binding spot <author here>.One key difference found was a point mutation at position 7 between an Isoleucine and Threonine [1], which would result in a higher affinity for the tirzepatide molecule binding onto the receptor than the ligand.
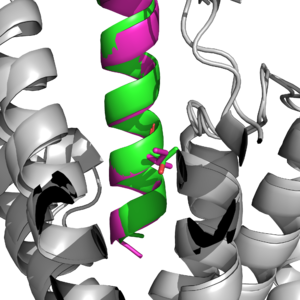
Figure 2: Key difference between GIP ligand and Tirzepatide at position 7. Ile7 is in pink (ligand), and Thr7 is in aqua(tirzepatide).
References
- ↑ 1.0 1.1 Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS, Coghlan MP, Kobilka BK, Sloop KW. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2116506119. PMID:35333651 doi:10.1073/pnas.2116506119
- ↑ Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules. 2022 Jul 5;27(13):4315. PMID:35807558 doi:10.3390/molecules27134315
Student Contributors
SaraKathryn Kalkhoff
Camille Gaudet


