User:Jaelin Lunato/Sandbox 1
From Proteopedia
Amylin Receptor (AMYR)
History and DiscoveryIn the early 1900s, amyloid protein deposits were found in the pancreatic tissue of diabetic patients. Later in 1999, the structure of the family of calcitonin receptors started to be determined, and it was determined that the amylin receptor (AMYR[1]) had a core structure of the calcitonin receptor (CTR). Additionally, it was determined that AMYR had a receptor activity-modifying protein (RAMP) bound to the CTR core. In 2000, the CTR and a RAMP was co-expressed in a single cell, and later in 2016 the CTR and a RAMP were both found in rat brain cells. Cryo-electron microscopy (cryo-EM) was used to determine the structure of the calcitonin receptor and later the structure of AMYR. NMR and x-ray crystallography was also used to determine the structure but the resolution was poor relative to cryo-EM. StructureCellular Domains AMYR has three domains: extracellular, transmembrane, and intracellular. The CTR and the RAMP have both and . The calcitonin receptor is a 7-pass helix chain to which RAMP binds to become the amylin receptor [2]. The ligand, amylin, binds within the transmembrane domain. The G protein is located . The location of each component of the amylin receptor is essential in determining structure and function. For example, it is important for the G protein to be located inside the cell in order to initiate a signal cascade for cell signaling.
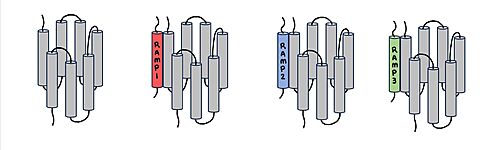
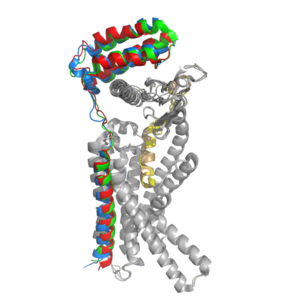
CTR and RAMP Heterodimer AMYR is a heterodimer of a calcitonin receptor and a receptor activity-modifying protein. There are three different RAMPs, RAMP1, RAMP2, and RAMP3, that compose AMY1R, AMY2R, and AMY3R when associated with the CTR (Figure 1). The three different RAMPs are structurally similar to each other, so all three RAMPs are able to bind to the CTR without any modification of the CTR (Figure 2) [2].
In the absence of a RAMP, the calcitonin receptor has greater affinity for calcitonin than amylin, but because the , both calcitonin and amylin can bind to the CTR without any modification of the receptor [2]. The two ligands have many conserved residues (highlighted in blue), and this shows that the two ligands share many chemical properties which explains why they are structurally similar. In addition to having many conserved residues, both ligands also have an amidated C-terminus (Figure 3). When the CTR is bound to a RAMP, the complex becomes the AMYR and has greater affinity for the relative to the calcitonin ligand. Therefore, the RAMP is essential to AMYR because it causes AMYR to have greater affinity for the amylin ligand rather than the calcitonin ligand. LigandsThe two major ligands of the calcitonin receptor are calcitonin and amylin. Calcitonin is a peptide hormone secreted from the thyroid, and it is involved in the regulation of calcium and phosphate in the blood. Amylin is also a hormone and is secreted by pancreatic beta cells. Amylin binding activates many different biological processes affecting different systems with the human body. For example, amylin binding can affect the immune system, the central nervous system, and the satiation system in the brain (i.e., the area postrema). There are two required post-translational modifications of amylin in order for the ligand to have any bioactivity: (1) and (2) a between C2 and C7. The amidated C-terminus of amylin has a nitrogen present which makes three significant hydrogen bonds. There is one hydrogen bond between the end of amylin and the backbone of the CTR and two hydrogen bonds back to the backbone of amylin itself. The amidated C-terminus is essential to the bioactivity of amylin because the Nitrogen participates in Hydrogen bonding and holds the amylin ligand in a specific orientation. Additionally, the hydrogen bonds orient the amylin Y37 residue in a specific orientation so the Y37 side chain can participate in non-polar interactions in the space opposite of the hydrogen bonds. The disulfide bonds between C2 and C7 inhibit the end of amylin from waving around freely, and it prevents amylin from aggregation as well. 12 Angstrom Calcitonin ShiftWhen the RAMP binds to the CTR, the CTR undergoes a conformational change, or shift. The extracellular region of the calcitonin subunit, specifically in the C-alpha loop 5, of AMYR will undergo a 12 angstrom shift upwards. This distance shift is measured specifically from a glutamate residue, E123. This shift in the CTR facilitates the packing of the CTR extracellular domain with the antiparallel triple helix conformation of the RAMP extracellular domain.
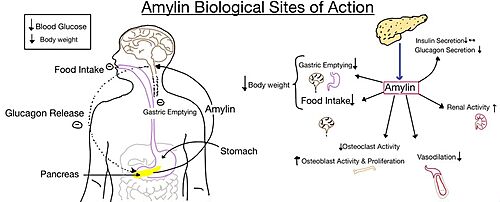
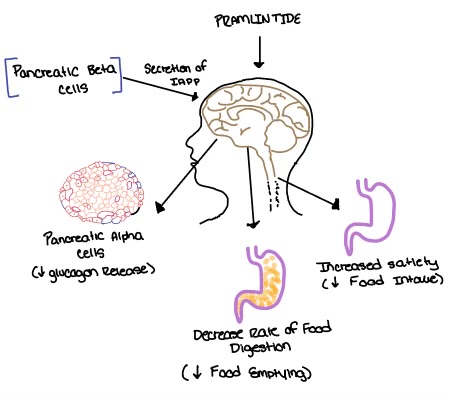
There is little movement in the N-terminal location and midregion of the CTR when RAMP binds. This is because there is an unstructured and flexible "hinge" region between the extracellular domain and the core-binding domains, allowing these domains to remain in their original location from before RAMP binds to the CTR. Binding Site InteractionsThere are . These bonds contribute to the functional phenotype of AMYR and also causes the end of the amylin ligand to be held in a flipped up position. There are water molecules present in the binding site between amylin and the calcitonin receptor that support the ligand-receptor interaction. Some water molecules interact with the amylin ligand and create water-bridged Hydrogen bonds between different ligand residues, such as the . Other water molecules create . The water molecules are present in the empty space located in the ligand binding site, and they are hypothesized to stabilize the active conformation of the calcitonin receptor when amylin is bound. Substitutions of polar residues involved with the water-bridged Hydrogen bond network to nonpolar residues causes a decrease in potency and affinity of amylin to the calcitonin receptor. (REFERENCE NEEDED HERE******) G Protein ActivationThere are extensive nonpolar, . Val322 and Leu323 of the CTR 2nd intracellular loop makes nonpolar interactions with Ile248 and Val249 back to itself as well as Leu388 of the Gα subunit. These interactions stabilize the calcitonin receptor, which activates the G-protein to give amylin its biological role. The . Arg180 of the 3rd intracellular loop of the calcitonin receptor makes a crucial hydrogen bond to Gln384 of the Gα subunit. This interaction has been shown to maintain the biological function of amylin through cell signaling (REFERENCE NEEDED HERE*******). These interactions between the second and third intracellular loops of the calcitonin receptor stabilize the conformational states of the G-protein to create a more rigid domain with the nanobody. Biological RelevanceThe functional pharmacology of AMYRs has relied on interference from differences between the behavior of CTRs in the presence and absence of RAMPs [2]. Thus, understanding the structural basis for binding and selectivity of peptides to CTR and AMYRs is important for future drug discovery and development. DiabetesAmylin, as it is a part of the calcitonin peptide family, is heavily related to the regulation of homeostatic processes to relevant drug targets. Amylin is the target for the treatment of diabetes. Amylin is a neuroendocrine hormone that is synthesized and co-secreted with insulin. Insulin triggers glucose uptake which removes glucose from the bloodstream using it then for energy. Amylin works in negatively regulating (inhibiting) the formation of glucagon so that glucose polymers can continue to be broken down into the bloodstream for further energy storage and consumption. Therefore with the co-secretion of both amylin and insulin, it would aid in decreasing blood glucose levels thus becoming a predominant treatment plan for diabetic disorders, such as shown with the developing drug Pramlintide. PramlintidePramlintide, a peptide analog of human amylin, is FDA-approved for the treatment of insulin-requiring diabetes (Figure 5) [2]. Pramlintide is injected into the bloodstream by the beta cells of the pancreas along with insulin after a meal, aiding in the regulation of blood glucose by slowing gastric emptying, promoting satiety via hypothalamic receptors, and inhibiting secretion of glucagon which opposes the effects of insulin and amylin (Figure 6) [3].
Alzheimer's
| ||||||||||||
References
- ↑ Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, Christopoulos A, Hay DL, Danev R, Wootten D, Sexton PM. A structural basis for amylin receptor phenotype. Science. 2022 Mar 25;375(6587):eabm9609. PMID:35324283 doi:10.1126/science.abm9609
- ↑ 2.0 2.1 2.2 2.3 2.4 Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev. 2015 Jul;67(3):564-600. PMID:26071095 doi:10.1124/pr.115.010629
- ↑ Thapa, G., Kumari, A., Dasgupta, D., Bandyopadhy, S., Sarkar, N., Roy, K., Karunakaran, G., Kazmi, I., Karmakar, S., & Chakraborty, M. (2023). Chapter 5- Insight into the mechanism of action of anti-diabetic drugs. How Synthetic Drugs Work. 95-122. DOI:10.1016/B978-0-323-99855-0.00005-1
- ↑ Press, M., Jung, T., Konig, J., Grune, T., & Hohn, A. (2019). Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mechanisms of Ageing and Development. 3, 46-54. DOI:10.1016/j.mad.2018.03.010