This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Leaf branch compost cutinase (LCC) is a versatile enzyme that is capable of breaking down both natural plant polymers and synthetic plastics.[3] It was discovered in a compost heap, and it originally evolved to degrade cutin, the protective biopolymer in plant surfaces. LCC has also shown high efficiency in hydrolyzing polyethylene terephthalate (PET), which is a widely used plastic that contributes to global pollution. Unlike many other PET-degrading enzymes, LCC is both thermostable and has a high catalytic efficiency, which means it can function at temperatures that are optimal for industrial recycling processes. By breaking PET into its monomers, LCC enables the closed-loop recycling of plastic waste and reduces environmental accumulation. Research is focused on engineering LCC variants with improved activity and thermostability to accelerate plastic degradation.
[4]
[5]
Function
LCC catalyzes the hydrolysis of the ester bonds in PET polymers and breaks them down into their constituent monomers: terephthalic acid and ethylene glycol. The enzyme operates through a catalytic triad consisting of three amino acid residues: S165, D210, and H242. These residues enable LCC to perform nucleophilic attacks on the carbonyl carbon atoms of the ester bonds in PET. During catalysis, the substrate binds in an elongated, predominantly hydrophobic groove present in the enzyme's structure.
LCC functions optimally at elevated temperatures (around 65-72°C), which approaches the glass transition temperature of PET. This temperature range maximizes PET chain mobility and makes the polymer more accessible to enzymatic action. The enzyme is remarkably thermostable compared to other PET hydrolases, with a melting temperature of 84.7°C. This property allows it to remain functional under these high-temperature conditions. Also unlike other PET hydrolases such as Is-PETase, BTA1, BTA2, and FsC, LCC demonstrates substantially higher catalytic efficiency. Specifically, LCC achieves an initial PET-specific depolymerization rate of 93.2 mg TAeq. h⁻¹ mg⁻¹ enzyme at 65°C with amorphous PET. This means that it us at least 33 times more efficient than other tested enzymes.[3]
LCC's function is limited by PET crystallinity, as the enzyme more effectively hydrolyzes amorphous regions of the polymer. As PET crystallinity increases during the depolymerization reaction (due to exposure to elevated temperatures), the enzyme's efficiency decreases. This ultimately limits complete depolymerization unless optimal conditions and enzyme variants are used.
Relevance
With global plastic production reaching approximately 299 million tons annually, the need for effective waste management solutions has become urgent. Enzymatic degradation is an alternative to conventional recycling methods that are often inefficient and environmentally taxing. One of the primary challenges in plastic waste management is the volume of mismanaged plastic entering marine environments. In 2010 alone, an estimated 31.9 million metric tons of plastic waste were classified as mismanaged, with a substantial portion ending up in the oceans. This causes harm to marine ecosystems, physical injury to wildlife, and disruption of food chains.[6]
Integrating LCC into existing waste management systems could substantially reduce the PET waste that enters the environment. Research suggests that a 77% reduction in mismanaged plastic waste could lower the annual input of plastic into the ocean to between 2.4 and 6.4 million metric tons by 2025.
LCC hydrolyzes PET into its constituent monomers, which also supports the principles of a circular economy, where materials are reused rather than discarded. Beyond degradation, enzymatic degradation allows for the production of biologically recycled PET with properties that are comparable to virgin materials.[7]
Structural Overview
Catalytic Triad
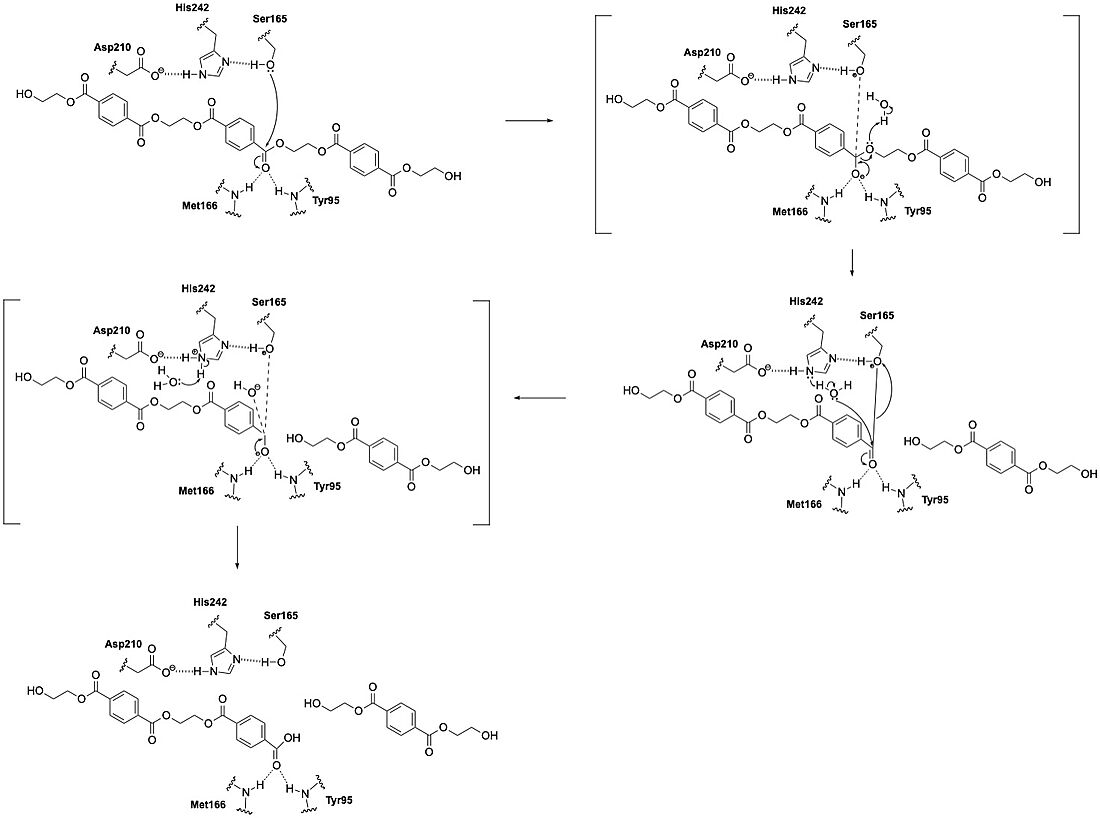
As the PET substrate binds to the enzyme, its carbonyl bond
attached to the first benzene ring must be harbored close to Serine in
the catalytic triad (Fig. 2). Serine gets polarized by histidine, which is
then stabilized by aspartic acid. Polarized Serine will attack the
carbonyl bond (C-O) of the polyester, resulting in a tetrahedral in-
termediate, which is then stabilized by the oxyanion hole. The hy-
drolysis procedure is completed by a second nucleophilic attack
mediated through a water molecule "PEThydrolase-insilico-engineering"
Ligand Binding Site
hydrophobic pocket from "An engineered PET depolymerase to break down and recycle plastic bottles"
Mutations Sites of Interest
F243
F243W
T96
Y127
N246
S283 & D238
It gains stability with two disulfide bonds, while only
one conserved disulfide bond exists in other homologs. Conserved
disulfide bond C273-C289 connects the last loop to the C-terminal
helix. The IsPETase-specific disulfide bond C203-C239 harbors the
catalytic acid and the base [43], and has been shown to result in a
lower energy barrier/higher efficiency for PET hydrolysis - "PEThydrolase-insilico-engineering"
Key residues that were on the
metal site of known homolog enzymes were identified as D238 and
S283. They mutated by cysteine to introduce a disulfide bonding to
increase melting temperature instead of metal ion stabilization. The
engineered LCC version named LCC-ICCG (with D238C/S283C along
with F243I and Y127G) is reported to acieve 90% of PET depoly-
merization. Recent study by Zeng and coworkers reported an in-
crease in the melting temperature of LCC-ICCG to 98.9 °C by addition
of A59K, V63I, and N248P mutations. However, the optimal hydro-
lyzing temperature was found to be 74 °C [58]. -"PEThydrolase-insilico-engineering"


