Function
Background
P2X receptors are a family of ligand‐gated ion channels that open in response to extracellular adenosine triphosphate (ATP). They mediate rapid purinergic signaling by allowing cation flux (Na⁺, K⁺, and Ca²⁺) across the plasma membrane. Seven mammalian P2X subunits (P2X₁–P2X₇) assemble as homo‐ or heterotrimers to form channels with distinct kinetic and pharmacological properties[1].
Structure and Function
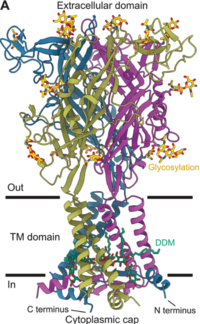
Each P2X subunit is ~380 amino acids long, featuring two helices (TM1 and TM2), a large extracellular , and intracellular N‐ and C‐termini. Upon assembly, three subunits arrange around a central pore. ATP binds at intersubunit clefts in the extracellular domain, inducing conformational changes that open the pore[1].
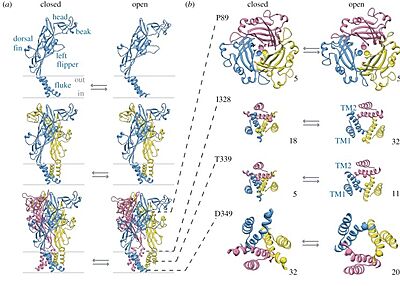
Open/closed ion channel of
9bqi. Adapted from North (2016)
[1].
Binding of two to three ATP molecules triggers channel opening within milliseconds, permitting rapid cation influx. The initial current is predominantly Na⁺ and Ca²⁺ inward current, followed by a slower “desensitization” or “pore dilation” phase in certain subtypes (notably P2X₇), which can lead to larger pore formation and prolonged permeability to larger cations.
P2X receptors are widely expressed in nervous, immune, and cardiovascular systems. Key functions include: Neurotransmission: P2X₃ in sensory neurons mediates pain perception.
Inflammation and immune response: P2X₇ activation promotes cytokine release and cell death in macrophages. Muscle contraction: P2X₁ in smooth muscle contributes to vasoconstriction and bladder control. Bone remodeling: P2X₇ in osteoblasts and osteoclasts regulates bone turnover[2].
Structural insights from cryo-EM studies
Cryo-electron microscopy of full-length human P2X₄ has suggested how intracellular elements and lipids shape gating and desensitization:
Preformed cytoplasmic cap: Structures of the apo-closed and antagonist-bound inhibited states reveal an intact “” prior to ATP binding, indicating that cap formation precedes activation rather than resulting from it.
Lipid stabilization of desensitization: Functional assays and density for suggest that specific lipid–protein interactions stabilize the cytoplasmic cap, slowing the transition to the desensitized state and thus modulating receptor responsiveness.
Post-translational modifications: P2X₄ is decorated by in the extracellular vestibule and palmitoylation on intracellular residues, modifications that likely influence trafficking, lipid interactions, and gating dynamics.
Unique allosteric pocket: The -bound inhibited structure uncovers a human-specific allosteric ligand-binding pocket at the subunit interface, offering a template for design of subtype-selective small-molecule modulators of P2X₄ and potentially other P2X receptors.
Publication Abstract
P2X receptors (P2XRs) are adenosine 5'-triphosphate (ATP)-gated ion channels comprising homomeric and heteromeric trimers of seven subtypes (P2X1-P2X7) that confer different rates of desensitization. The helical recoil model of P2XR desensitization proposes stability of the cytoplasmic cap sets the rate of desensitization, but timing of its formation is unclear for slow-desensitizing P2XRs. We report cryo-electron microscopy structures of full-length wild-type human P2X4 receptor in apo closed, antagonist-bound inhibited, and ATP-bound desensitized states. Because the apo closed and antagonist-bound inhibited state structures of this slow-desensitizing P2XR include an intact cytoplasmic cap while the ATP-bound desensitized state structure does not, the cytoplasmic cap is formed before agonist binding. Furthermore, structural and functional data suggest the cytoplasmic cap is stabilized by lipids to modulate desensitization, and P2X4 is modified by glycosylation and palmitoylation. Last, our antagonist-bound inhibited state structure reveals features specific to the allosteric ligand-binding pocket in human receptors that facilitates development of small-molecule modulators.
Human P2X4 receptor gating is modulated by a stable cytoplasmic cap and a unique allosteric pocket.,Shi H, Ditter IA, Oken AC, Mansoor SE Sci Adv. 2025 Jan 17;11(3):eadr3315. doi: 10.1126/sciadv.adr3315. Epub 2025 Jan , 17. PMID:39823330[3]
MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
Relevance
Studying P2X receptors is of broad relevance because these channels sit at the nexus of extracellular ATP signaling and rapid cellular responses in virtually every organ system. By mediating cation flux in response to ATP, P2X receptors regulate synaptic transmission, sensory perception, and immune activation on the scale of milliseconds. Dysfunction of P2X signaling underlies pathologies as diverse as chronic pain, neuroinflammation, and hypertension. A deep understanding of P2X receptor structure–function relationships therefore not only illuminates fundamental mechanisms of purinergic signaling but also identifies molecular gateways for therapeutic intervention.