This is a default text for your page Vinícius M. Neto/Sandbox 1. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function
Basic structure
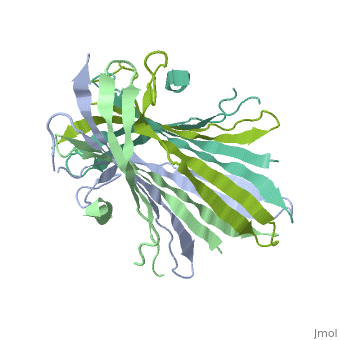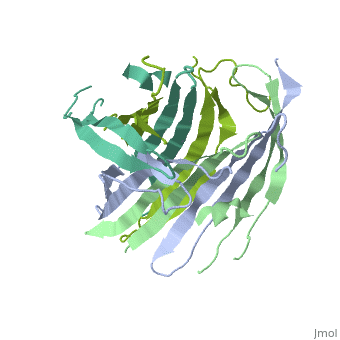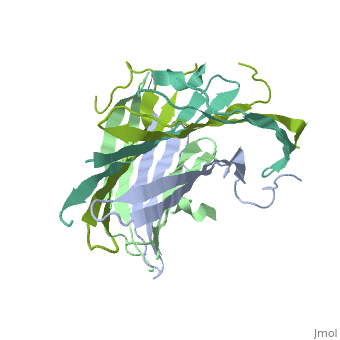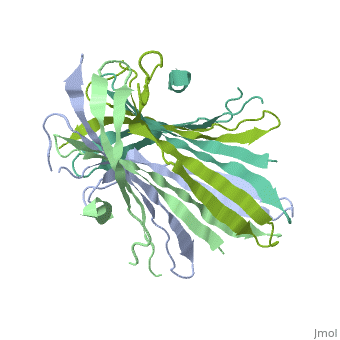
The N-terminal domain of the fibroin heavy chain (FibNT 3UA0) is a homo-tetramer composed of 268 residues, most of which are (hydrophilic amino acids in marron and hydrophobic in medium blue). FibNT's asymmetric unit is a homodimer with eight alternating β-sheets and a disordered C-terminus (Gly109-Ser126). Its two chains (A and B) are nearly identical except for the N-terminal segments (Phe26-Val35):
- Chain A: Forms a short α-helix.
- Chain B: Adopts a loop conformation.
The FibNT homodimer exhibits the following topology: β1A–β2A–β4B–β3B–β3A–β4A–β2B–β1B, where the β-sheets are connected via two β-hairpins (Thr36–Asn65 and Glu78–Ser107) and two type I β-turns (Asp49–Gly52 and Asp89–Gly92). The entire assembly is stabilized by an extensive network of hydrogen bonds between adjacent β-strands.
Oligomerization
Fibroin oligomerization is deeply afected by the pH. Naturaly, during the silk spinning process, the fiber is subjected to a decreasing pH gradient from the anterior to the posterior part of the silk gland, which triggers the gelation of the condensed fibroin. In particular, the FibNT exists in a random coil state, which prevents premature formation of β-sheets. As the pH decreases to around 6.0, FibNT undergoes a structural transition to form β-sheets.
The interation between the acidic residues of FibNT may play an important role in this behavior. Some of these may have their pKa values up-shifted near the transition point and therefore ionize at neutral pH. This would hinder the protein folding and assembly processes due to charge repulsion. For instance, the hydrogen bonds formed by Glu56 and Asp100 with Asp44 and Glu98 respectively could be broken in higher pH environements, destabilizing the β-sheets conformations.
Relevance
Silk fibers from Bombyx mori have been utilized by mankind since ancient times due to its remarkable mechanical properties and comfort when woven into fabrics, its earliest record being around 2.700 BC. It is thermally comfortable, elastic, strong and soft and that is why it is highly sought after as a luxury fabric for garments. It is also biocompatible, making it even applicable as a medical biomaterial for sutures or scaffolds.
Bombyx mori silkworms naturally use their silk to construct a cocoon at the end of the final stage of larval development before cocoon formation, before they undergo metamorphosis into a moth. During the natural spinning process, the silkworm extrudes the silk dope (a water-soluble liquid crystalline state containing up to 30%wt/vol fibroin in water) from its spinnerets into the external environment. This process involves mechanical shearing, stretching, and water evaporation. The delicate gland conditions (silk dope acidification, concentration changes of metal ions, and water content reduction) are crucial for the proper folding of fibroin into micelles and then liquid crystals.