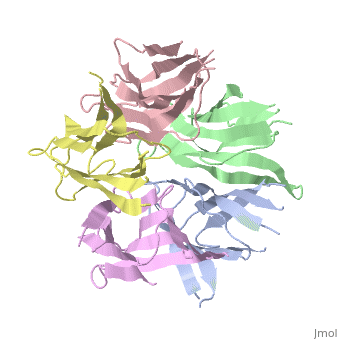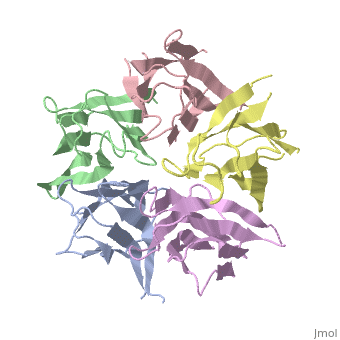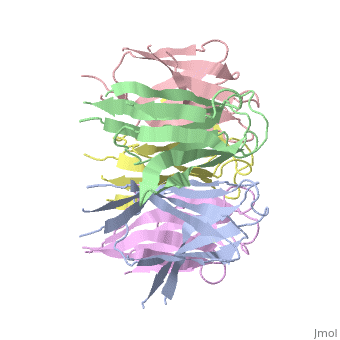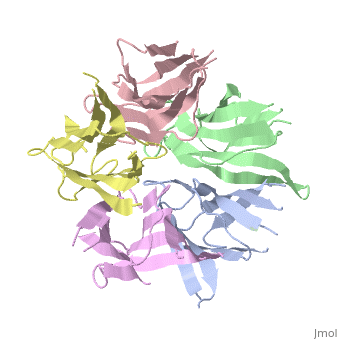1k5j
From Proteopedia
|
The Crystal Structure of Nucleoplasmin-Core
Overview
The efficient assembly of histone complexes and nucleosomes requires the, participation of molecular chaperones. Currently, there is a paucity of, data on their mechanism of action. We now present the structure of an, N-terminal domain of nucleoplasmin (Np-core) at 2.3 A resolution. The, Np-core monomer is an eight-stranded beta barrel that fits snugly within a, stable pentamer. In the crystal, two pentamers associate to form a, decamer. We show that both Np and Np-core are competent to assemble large, complexes that contain the four core histones. Further experiments and, modeling suggest that these complexes each contain five histone octamers, which dock to a central Np decamer. This work has important ramifications, for models of histone storage, sperm chromatin decondensation, and, nucleosome assembly.
About this Structure
1K5J is a Single protein structure of sequence from Xenopus laevis. The following page contains interesting information on the relation of 1K5J with [Importins]. Full crystallographic information is available from OCA.
Reference
The crystal structure of nucleoplasmin-core: implications for histone binding and nucleosome assembly., Dutta S, Akey IV, Dingwall C, Hartman KL, Laue T, Nolte RT, Head JF, Akey CW, Mol Cell. 2001 Oct;8(4):841-53. PMID:11684019
Page seeded by OCA on Sun Nov 18 09:02:40 2007
Categories: Importins | Single protein | Xenopus laevis | Akey, C.W. | Akey, I.V. | Dingwall, C. | Dutta, S. | Hartman, K.L. | Head, J.F. | Laue, T. | Nolte, R.T. | Beta-barrel | Beta-bulge | Jellyroll | Pentamer