Acetylcholinesterase
From Proteopedia
|
3D structure of acetylcholinesterase
Contents |
Key Enzyme in the Nervous System
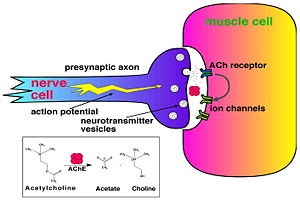
Acetylcholinesterase (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, acetylcholine (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors.
The 3D structure of Torpedo californica AChE (TcAChE) (Sussman et al. & Silman (1991)) opened up new horizons in research on an enzyme that had already been the subject of intensive investigation. The unanticipated structure of this extremely rapid enzyme, in which the active site was found to be buried at the bottom of a , lined by aromatic residues, led to a revision of the views then held concerning substrate traffic, recognition, and hydrolysis (Botti et al. Sussman & Silman (1999)).
Alzheimer’s disease (AD) is a debilitating brain disease that occurs in around 10% of the elderly and, as yet, there is no known cure. At present, the most widely used treatments consist are medications that attempt to increase the brain’s levels of ACh, whose levels decrease with onset of disease. These drugs work by interfering with AChE. Thus drugs that are mild inhibitors of AChE, like Tacrine, E2020 (Aricept) and the Traditonal Chinese Medicine (TCM) Huperzine appear to retard symptoms of AD.
|
The active site gorge has , a catalytic site (consisting of the catalytic triad together with Trp84 & Phe330) and a peripheral site (including Trp 279 & Tyr 121), which helps prebind the substrate and direct it toward the active site. The 3D structure showed not only that the active site was buried deep in the enzyme, but surprisingly, there were no negatively charged residues along this gorge, as was expected to help attract the positively charged ACh substrate, rather, instead, a series of aromatic residues that are highly conserved in all AChE sequences. See: AChE inhibitors and substrates
Selected 3D Structures of AChE
- 2ace This is the original solved structure for T. californica
- 1ea5 This is the highest resolution X-ray structure of AChE determined till now.
- 1eve The E2020 (Aricept) complex.
- 1ax9 Edrophonium complex.
- 1vot Complex with huperzine, a traditional Chinese folk medicine.
- 1fss Complex with the snake venom toxin, Fasciculin-II.
- 1vzj Model complex of the cholinesterase tetramer.
- 1som Complex with nerve agent soman (GD).
More structures can be obtained by searching for AChE
|
Flexibility of aromatic residues in the active-gorge of AChE
The high aromatic content of the deep and narrow active-site gorge of acetylcholinesterase (AChE) is a remarkable feature of this enzyme.There are lined along the gorge of Torpedo californica AChE (TcAChE), F120, F288, F290, F330, F331, W84, W233, W279, W432, Y70, Y121, Y130, Y334, and Y442. The side-chain conformational analyses based on the multuple available crystal structures and molecular dyanmics (MD) simulation trajectories show that the degree of flexibility of these 14 aromatic side chains is diverse. While those of are both very flexible, the side-chain conformations of appear to be fixed. Residues located on, or adjacent to the , viz. , display different flexibilities in the MD simulations and in the crystal structures.
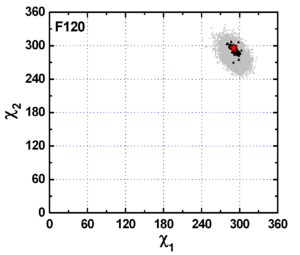
Group I: residues with fixed side-chain conformation
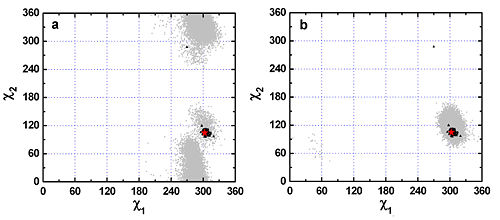
F120 as well as W233, W432, Y70, Y121, F331, F288, and F290 fall into group I, the category of residues with fixed side-chain conformations in both crystal structures and MD simulation trajectory. Fig. 1 shows that both the experimental and MD data are concentrated in a specific region. In this figure, the grey dots are derived from a 20-ns MD trajectory of native TcAChE and each one represents a pair of χ1 and χ2 angles calculated based on snapshot structures of TcAChE extracted from the MD trajectory at 1 ps intervals. The plot contains 20,000 such dots in total. The red pentacle is the crystal structure (pdb code 1ea5) used for the MD simulation. The dark triangles present pairs of c1 and c2 angles calculated based on 89 crystal structures of AChE deposited in the PDB.
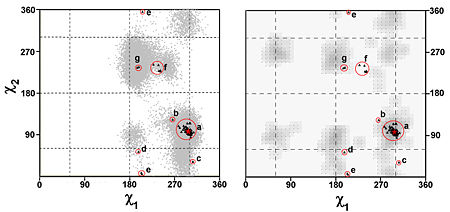
Group II: residues with flexible side chains
|
The side-chain flexibility of W279 is quite high in both data sets. The MD simulation of the native enzyme could produce most of side-chain conformations revealed by the crystal structures of complexes, suggesting that not the induced-fit but rather the preexisting equilibrium dynamics are involved in the conformational changes of W279 with regard to binding of these ligands. Moreover, it is striking that a standard χ1/χ2 rotomer plot of tryptophan would miss several of these regions both explored in MD simulation and crystal structures, such as group f (Figure 2).Rational drug design, based on structural information, might profit by taking into account conformational heterogeneity observed in MD simulations.
F330 is known for to have high side-chain flexibility as revealed by earlier experimental and simulation studies. The same conclusion was obtained in the current study. The only puzzle is that in some native structures F330 adopts an unfavored side-chain orientation according to PROCHECK. The re-examination of the electron density of these ‘strange’ native structures indicated that there may be a PEG bound in the active-site gorge of the structures in which the side-chain conformation of F330 is unfavorable.
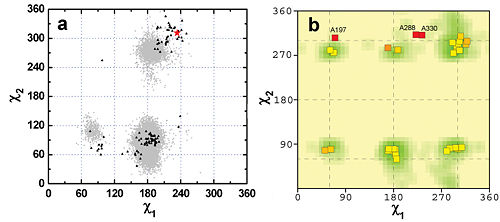
Group III: Residues with different degree of side-chain flexibility in MD simulations and crytal structures
W84 as well as Y130, Y442, and Y334 behave differently in crystal structures and in MD trajectories. Comparison of the two data sets revealed that not only the side-chains of the four residues but also the main-chain of the omega-loop (C67-C94) on which the four residues are located or are adjacent to are different. In all the crystal structures, the conformation of the omega-loop is quite fixed because of the crystal packing. In contrast, this loop is very flexible in the MD simulation. A 2nd MD simulation, with main-chain fixed, which mimics the effect of the crystal packing, however, produced side-chain conformations similar to those in the crystal structures (Fig. 4b).
Conclusions
The side-chain flexibility analyses of the 14 aromatic residues based on the multiple crystal structures, together with the MD simulation trajectories, could benefit structure-based drug design for AChE, and understanding of the dynamic properties of the active-site gorge as well as ligand trafficking within it. The good agreement between conformational sampling of the crystal structures and the MD simulations suggests that the latter is a valid method to explore the conformational landscape of the residues as well as of the whole protein.
Additional Resources
For additional information, see: Alzheimer's Disease
References
- [1] Yechun Xu, Jacques Philippe Colletier, Hualiang Jiang, Israel Silman, Joel L. Sussman, Martin Weik. Induced-fit of preexisting equilibrium dynamics? Lessons from protein crystallography and MD simulations on acetylcholinesterase and implications for structure-based drug design. Prot. Sci. 2008, 17, 601-605.
- [2] Yechun Xu, Jacques Philippe Colletier, Martin Weik, Hualiang Jiang, John Moult, Israel Silman, Joel L. Sussman. Flexibility of aromatic residues in the active-site gorge of acetylcholinesterase: X-ray vs MD. Biophys. J. 2008, 95, 2500-2511.
Ache_apo_14aromaticresidues
External Links
Acetylcholinesterase Tutorial by Karl Oberholser, Messiah College
PDB Molecule of the Month - Acetylcholinesterase
Movies: X-ray Damage in ACh & Nature's Vacuum Cleaner by R. Gillilan, Cornell Univ
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu