1osm
From Proteopedia
|
OSMOPORIN (OMPK36) FROM KLEBSIELLA PNEUMONIAE
Overview
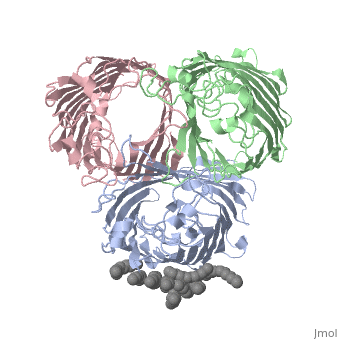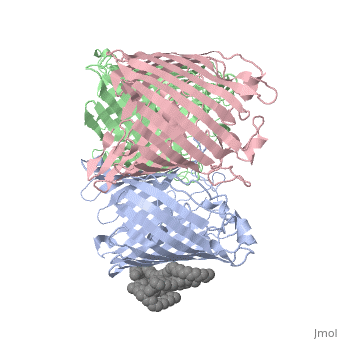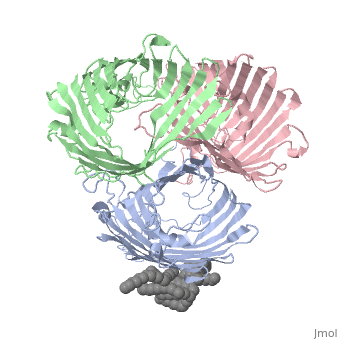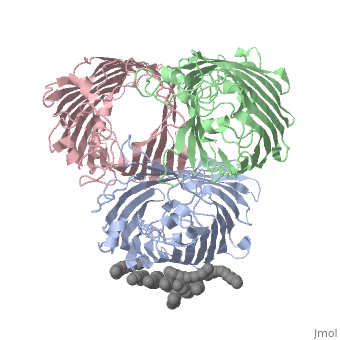
BACKGROUND: Porins are channel-forming membrane proteins that confer, solute permeability to the outer membrane of Gram-negative bacteria. In, Escherichia coli, major nonspecific porins are matrix porin (OmpF) and, osmoporin (OmpC), which show high sequence homology. In response to high, osmolarity of the medium, OmpC is expressed at the expense of OmpF porin., Here, we study osmoporin of the pathogenic Klebsiella pneumoniae (OmpK36), which shares 87% sequence identity with E. coliOmpC in an attempt to, establish why osmoporin is best suited to function at high osmotic, pressure. RESULTS: The crystal structure of OmpK36 has been determined to, a resolution of 3.2 A by molecular replacement with the model of OmpF. The, structure of OmpK36 closely resembles that of the search model. The, homotrimeric structure is composed of three hollow 16-stranded, antiparallel beta barrels, each delimiting a separate pore. Most, insertions and deletions with respect to OmpF are found in the loops that, protrude towards the cell exterior. A characteristic ten-residue insertion, in loop 4 contributes to the subunit interface. At the pore constriction, the replacement of an alanine by a tyrosine residue does not alter the, pore profile of OmpK36 in comparison with OmpF because of the different, course of the mainchain. Functionally, as characterized in lipid bilayers, and liposomes, OmpK36 resembles OmpC with decreased conductance and, increased cation selectivity in comparison with OmpF. CONCLUSIONS: The, osmoporin structure suggests that not an altered pore size but an increase, in charge density is the basis for the distinct physico-chemical, properties of this porin that are relevant for its preferential expression, at high osmotic strength.
About this Structure
1OSM is a Single protein structure of sequence from Klebsiella pneumoniae with D12 as ligand. Full crystallographic information is available from OCA.
Reference
Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae., Dutzler R, Rummel G, Alberti S, Hernandez-Alles S, Phale P, Rosenbusch J, Benedi V, Schirmer T, Structure. 1999 Apr 15;7(4):425-34. PMID:10196126
Page seeded by OCA on Tue Nov 20 23:09:36 2007