1rss
From Proteopedia
|
RIBOSOMAL PROTEIN S7 FROM THERMUS THERMOPHILUS
Overview
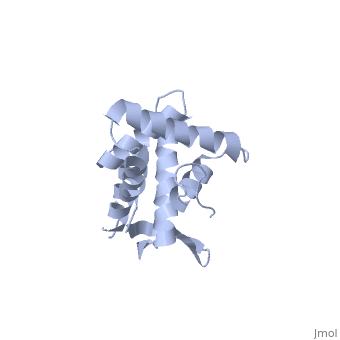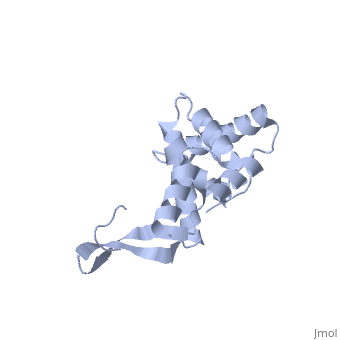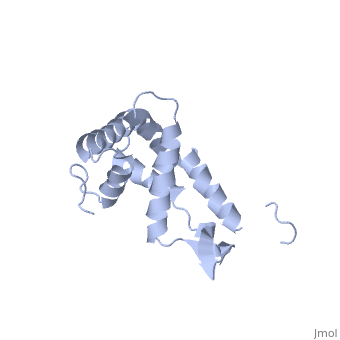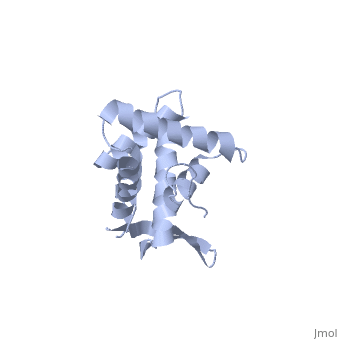
BACKGROUND: Ribosomal protein S7, a crucial RNA-binding component of the, ribosome, is one of two proteins that initiates assembly of the 30S, ribosomal subunit. It is required for proper folding of a large 3' domain, of 16S ribosomal RNA. S7 regulates its own synthesis by binding to its own, mRNA. This ability of S7 to bind both messenger and ribosomal RNAs makes, determination of its mode of RNA recognition particularly interesting., RESULTS: The crystal structure of S7 from Thermus thermophilus was, determined by a two-wavelength anomalous diffraction experiment using the, LIII edge of mercury. The S7 structure consists of a bundle of six helices, and an extended beta hairpin between helices 3 and 4, with two or more, RNA-binding sites on its surface. The hairpin, along with portions of, helices 1, 4 and 6, forms a large, positively charged, concave surface, that has the appropriate curvature and dimensions to bind double-stranded, RNA. A second putative RNA-binding site comprises parts of loop 2 and the, helix 4-loop 5 turn. CONCLUSIONS: Structural similarity between S7 and the, IHF/HU family of proteins strongly suggests that the beta hairpin of S7, binds to a groove of double-stranded RNA. The beta hairpin of S7 is also, similar to those from other nucleic acid binding proteins, such as, ribosomal protein L14 and BIV Tat, suggesting that it belongs to an, extended family of such motifs, all of which bind to a groove of, double-stranded nucleic acid. The residues in S7 loop 2 that belong to the, second putative RNA-binding site may have a role analogous to the, N-terminal residues of IHF/HU which grip an unbent portion of double, helix.
About this Structure
1RSS is a Single protein structure of sequence from Thermus thermophilus. Full crystallographic information is available from OCA.
Reference
The structure of ribosomal protein S7 at 1.9 A resolution reveals a beta-hairpin motif that binds double-stranded nucleic acids., Wimberly BT, White SW, Ramakrishnan V, Structure. 1997 Sep 15;5(9):1187-98. PMID:9331418
Page seeded by OCA on Wed Nov 21 01:50:49 2007