This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1v3v
From Proteopedia
|
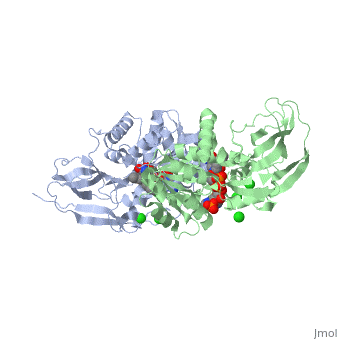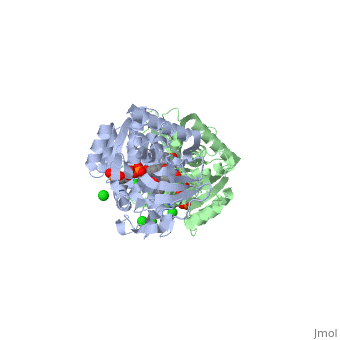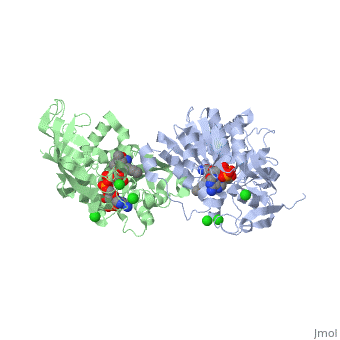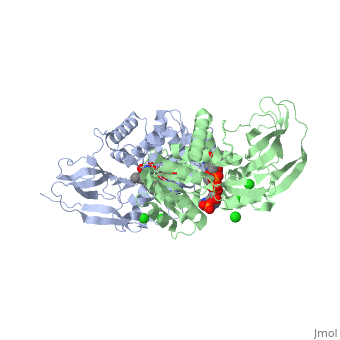
Crystal structure of leukotriene B4 12-hydroxydehydrogenase/15-oxo-prostaglandin 13-reductase complexed with NADP and 15-oxo-PGE2
Overview
The bifunctional leukotriene B(4), 12-hydroxydehydrogenase/15-oxo-prostaglandin 13-reductase (LTB(4), 12-HD/PGR) is an essential enzyme for eicosanoid inactivation. It is, involved in the metabolism of the E and F series of 15-oxo-prostaglandins, (15-oxo-PGs), leukotriene B(4) (LTB(4)), and 15-oxo-lipoxin A(4), (15-oxo-LXA(4)). Some nonsteroidal anti-inflammatory drugs (NSAIDs), which, primarily act as cyclooxygenase inhibitors also inhibit LTB(4) 12-HD/PGR, activity. Here we report the crystal structure of the LTB(4) 12-HD/PGR, the binary complex structure with NADP(+), and the ternary complex, structure with NADP(+) and 15-oxo-PGE(2). In the ternary complex, both in, the crystalline form and in solution, the enolate anion intermediate, accumulates as a brown chromophore. PGE(2) contains two chains, but only, the omega-chain of 15-oxo-PGE(2) was defined in the electron density map, in the ternary complex structure. The omega-chain was identified at the, hydrophobic pore on the dimer interface. The structure showed that the, 15-oxo group forms hydrogen bonds with the 2'-hydroxyl group of nicotine, amide ribose of NADP(+) and a bound water molecule to stabilize the, enolate intermediate during the reductase reaction. The electron-deficient, C13 atom of the conjugated enolate may be directly attacked by a hydride, from the NADPH nicotine amide in a stereospecific manner. The moderate, recognition of 15-oxo-PGE(2) is consistent with a broad substrate, specificity of LTB(4) 12-HD/PGR. The structure also implies that a Src, homology domain 3 may interact with the left-handed proline-rich helix at, the dimer interface and regulate LTB(4) 12-HD/PGR activity by disruption, of the substrate binding pore to accommodate the omega-chain.
About this Structure
1V3V is a Single protein structure of sequence from Cavia porcellus with CL, NAP and 5OP as ligands. Active as 15-oxoprostaglandin 13-oxidase, with EC number 1.3.1.48 Full crystallographic information is available from OCA.
Reference
Structural basis of leukotriene B4 12-hydroxydehydrogenase/15-Oxo-prostaglandin 13-reductase catalytic mechanism and a possible Src homology 3 domain binding loop., Hori T, Yokomizo T, Ago H, Sugahara M, Ueno G, Yamamoto M, Kumasaka T, Shimizu T, Miyano M, J Biol Chem. 2004 May 21;279(21):22615-23. Epub 2004 Mar 8. PMID:15007077
Page seeded by OCA on Wed Nov 21 04:25:32 2007
Categories: 15-oxoprostaglandin 13-oxidase | Cavia porcellus | Single protein | Ago, H. | Hori, T. | Kumasaka, T. | Miyano, M. | RSGI, RIKEN.Structural.Genomics/Proteomics.Initiative. | Shimizu, T. | Sugahara, M. | Ueno, G. | Yamamoto, M. | Yokomizo, T. | 5OP | CL | NAP | Riken structural genomics/proteomics initiative | Rossmann fold | Rsgi | Structural genomics