Introduction
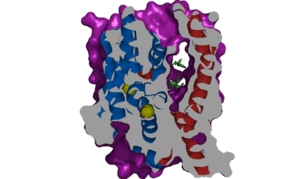
Structure
Binding Sites
Sodium
NTCP, among others in the SLC10 family, have . Many polar and negatively charged residues are characteristic of these active sites. The high level of conservation among sodium binding placement and interacting residues suggests sodium binding is coupled to bile salt transport. Additional mutations in the X-motif near sodium binding sites have shown that bile salt transport function is lost also suggesting that sodium allows bile salt binding.
[1] It is understood that these sodium binding sites facilitate changes from open-pore to closed pore states of NTCP that allow for the binding or release of bile salts. Closed-pore state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. This also allows for sodium concentrations to regulate uptake of taurocholates. When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, closed-state is favored preventing diffusion of taurocholates. [1]
Bile Salt
The is also characteristic of NTCP. The pore surface remains Hydrophobic, while lining of the open pore state is largely Polar. This pattern is believed to follow similar amphipathic patterns within taurocholate and other NTCP substrates, such as steroids or hormones [2] Thus the channel provides specificity while preventing leakage of other substrates. When observing the relevant it is shown that some residues form Van der Waals interactions while others will form dipole-dipole or ionic interactions with bile salt substrates. The core domain appears to contribute most of the polar domains, while the panel domain contributes more hydrophobic residues.
Conformational Change
| | 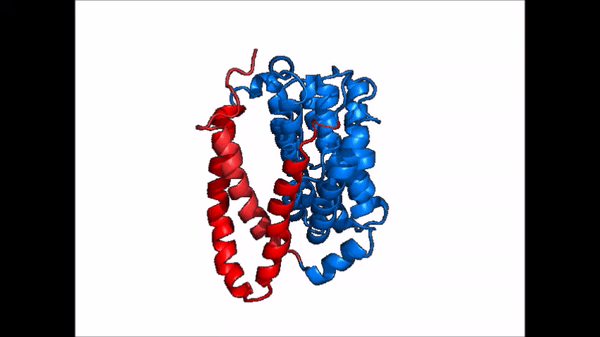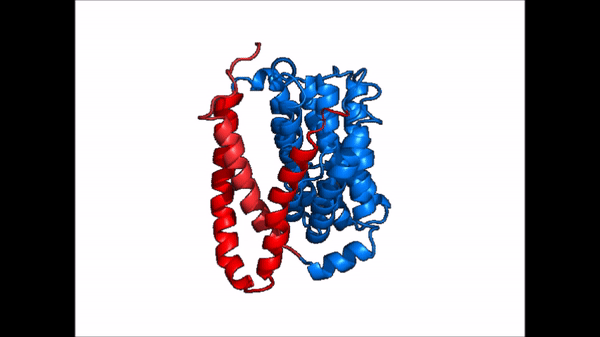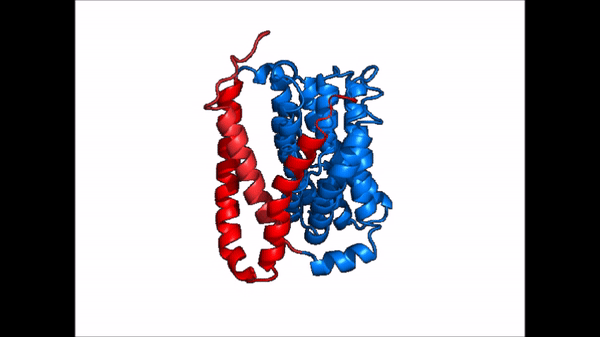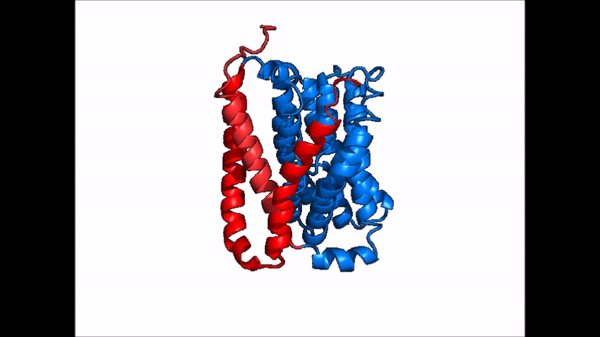 |
| Cartoon representation of NTCP conformational change. |
| | 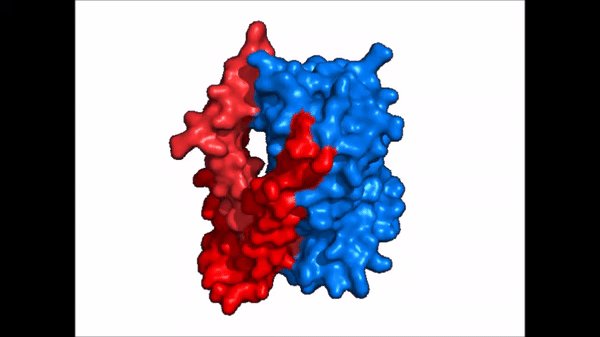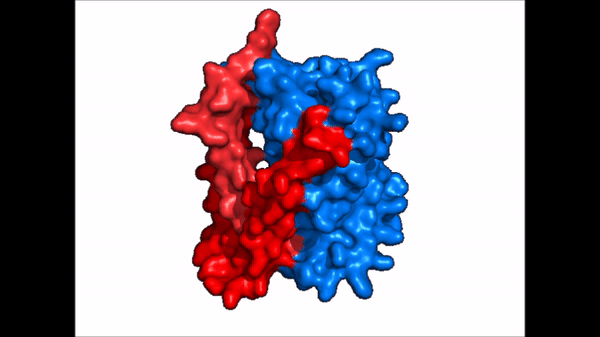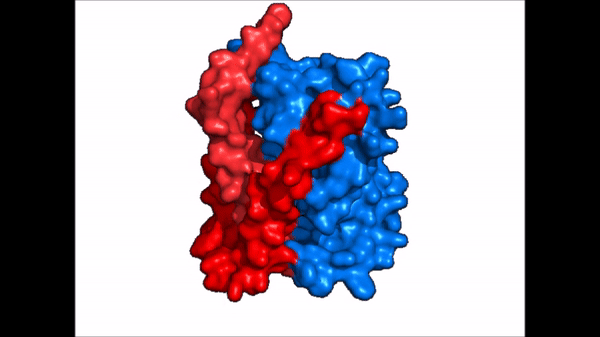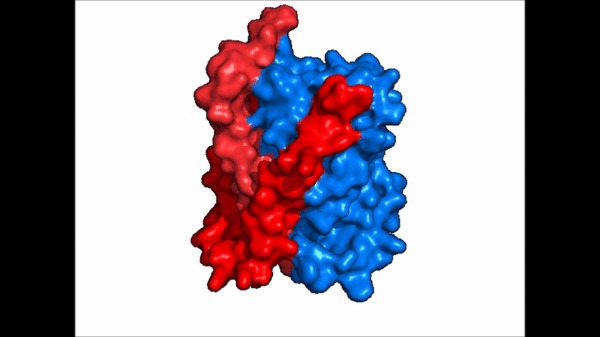 |
| Cartoon representation of NTCP conformational change. |
Bile Salt Transport
A proposed pathway for NTCP bile salt transport suggests that both sodium ions are translocated with the transport of one bile salt.[3] In this mechanism both sodium ions are released along with the inner bile salt into the cytoplasm (Fig. 5). The outermost bile salt remains bound however in the pore, likely helping to prevent leakage [3]