CRYSTAL STRUCTURE OF YAGE, A PROPHAGE PROTEIN BELONGING TO THE DIHYDRODIPICOLINIC ACID SYNTHASE FAMILY FROM E. COLI K12
One approach of the incorporation of genetic material from different species into bacterial genomes is infection by a bacteriophage. The yagE gene is located in the E. coli K12 genome and it is part of prophage CP4-6 encoding a 33-kDa putative dihydrodipicolinate synthase or 4-hydroxy-tetrahydrodihydrodipicolinate synthase (DHDPS)-like protein (UniProtKB/Swiss-prot: P75682). This DHDPS-like domain is a member of the N-acetyl neuraminate lyase (NAL) subfamily comprises an 8-fold (TIM barrel) with a small C-terminal α-helical domain (Inter pro: IPR005263). Many enzymes possess this DHDPS domain (e.g. HBPHA, KDGA, NAL, DOGDH, and DHDPS). All these enzymes contain an 8-fold α/β barrel and a small C-terminal α-helical region. between orthorhombic (2v8z) and monoclinic (2v9d) crystal forms of YagE revealed striking similarity.
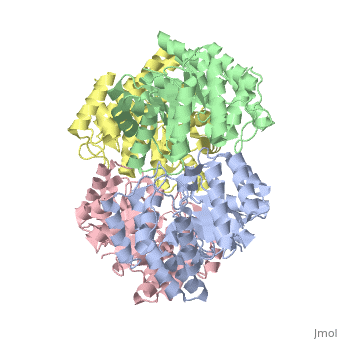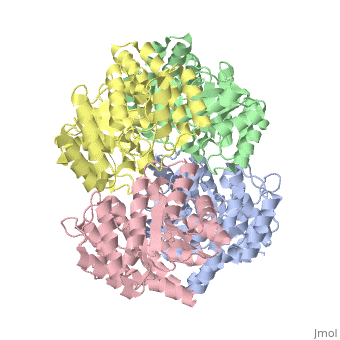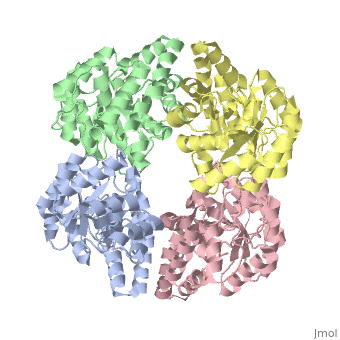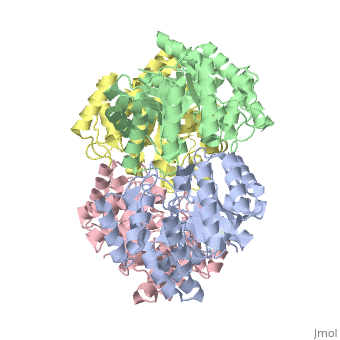
Cartoon representation of YagE showing the 2-fold symmetry axes between monomers. Chains A, B, C, and D are colored cyan, lime, magenta, and yellow, respectively. of YagE (cyan) with (1nal, yellow), (1w3i, magenta), (1xky, lime), and (2ats, blue) monomers.
Although YagE possesses only 23, 24, and 27% sequence identity with EcNAL, SsKDGA, EcDHDPS, respectively, their monomeric structures are all very similar.
Superposition of active site residues of YagE with (magenta, 1w3i), (blue, 2ats), and (yellow, 1nal), residues are labeled according to the corresponding PDB structures.
Members of the NAL protein subfamily have very similar active sites and a single amino acid substitution can significantly change their function. For example, NAL (1nal) gets DHDPS activity by substitution of a (orange) to (blue) at position 142. The possible active site region of YagE demonstrates closest sequence similarity to the active site of KDG aldolase of SsKDGA (1w3i) and NAL of EcNAL (1nal) (yellow), suggesting that this protein can perform either of these functions. Although the active site of EcDHDPS (1xky) and BaDHDPS (2ats) shows similarities, the important residue that differentiates between NAL and DHDPS, namely Leu142 (in 1nal), is also present in YagE (labeled cyan) at that particular position suggesting that YagE performs a NAL-related function rather than DHDPS-related one. In conclusion, the high-resolution X-ray structure of YagE provides a clue that it probably belongs to the NAL subfamily of proteins. Although the exact molecular function of YagE is still unknown, its structure provides a handle for understanding its molecular function based on knowledge about conserved residues of the putative active site.