Gamma Carbonic Anhydrase
From Proteopedia
| |||||||||||
Catalytic Mechanism
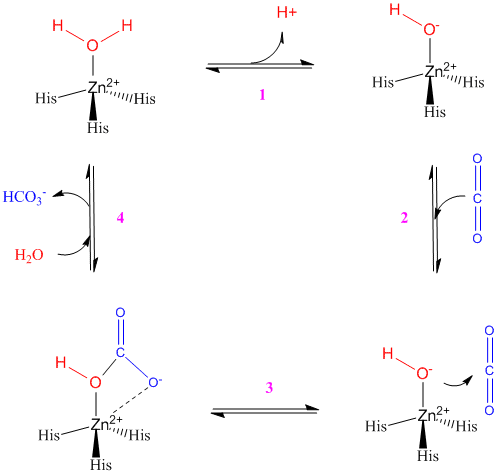
The catalytic mechanism for γ-carbonic anhydrase active site is shown below, using zinc as the metal ion ligand. In the case of cobalt as the metal ion ligand, there is an additional water molecule present in the active site. In the first step the zinc ion is coordinated with two water molecules (only one is shown on the diagram) and the three histidine residues. The Glu62 residue donates electrons to one of the water molecules, which in turn donates the electrons to the neighbouring water molecule (this is shown by the hydrogen ion on the diagram).The result is a zinc hydroxide. In the next step (2 to 3) the zinc hydroxide attacks the incoming carbon dioxide molecule, which is held in place in the proper orientation by Asn202 and Gln75 residues (not shown), to form the bicarbonate ion and displace one of the water molecules. In the final steps (3 to 4), the bicarbonate ion is displaced through hydrogen bonding with the Glu62 residue and the addition of two water molecules to the zinc ion. The water molecules replace the two oxygen molecules that were bound to the zinc ion, thus regenerating the enzyme [1] [4].
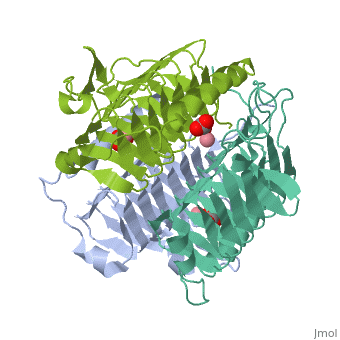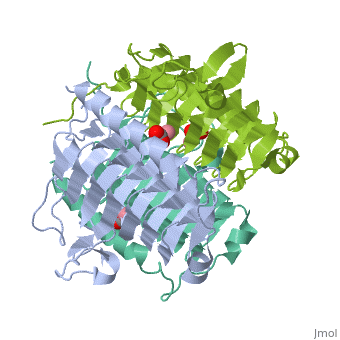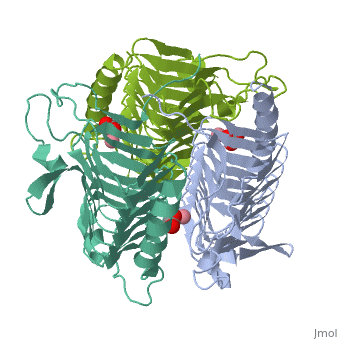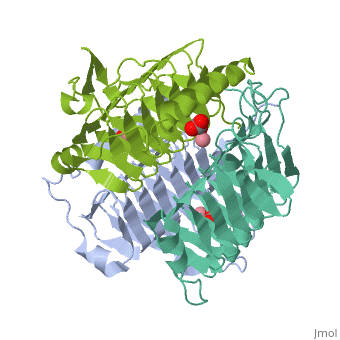
3D structues of γ-carbonic anhydrase
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Iverson TM, Alber BE, Kisker C, Ferry JG, Rees DC. A closer look at the active site of gamma-class carbonic anhydrases: high-resolution crystallographic studies of the carbonic anhydrase from Methanosarcina thermophila. Biochemistry. 2000 Aug 8;39(31):9222-31. PMID:10924115
- ↑ 2.0 2.1 Alber BE, Colangelo CM, Dong J, Stalhandske CM, Baird TT, Tu C, Fierke CA, Silverman DN, Scott RA, Ferry JG. Kinetic and spectroscopic characterization of the gamma-carbonic anhydrase from the methanoarchaeon Methanosarcina thermophila. Biochemistry. 1999 Oct 5;38(40):13119-28. PMID:10529183
- ↑ 3.0 3.1 3.2 Alber BE, Ferry JG. A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6909-13. PMID:8041719
- ↑ 4.0 4.1 Zimmerman SA, Ferry JG. Proposal for a hydrogen bond network in the active site of the prototypic gamma-class carbonic anhydrase. Biochemistry. 2006 Apr 25;45(16):5149-57. PMID:16618104 doi:10.1021/bi052507y