Sandbox Reserved 1850
From Proteopedia
| This Sandbox is Reserved from March 18 through September 1, 2025 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson and Mark Macbeth at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1828 through Sandbox Reserved 1846. |
To get started:
More help: Help:Editing |
Contents |
CE6
|
The DA_20_10 model of the Diels Alderase was further enhanced by players of the online game "Foldit." Building on preliminary early data, players were asked to optimize various helical structures that would surround and support the ligand. After over 100,000 designs were tested, the top-scoring CE6 model was finalized, containing as that favorably constrains ligand orientation. This "cap" consists of two helices--helix one spans from residues 36-44, and helix two spans from residues 48-56. [cite Eiben]
Kinetics
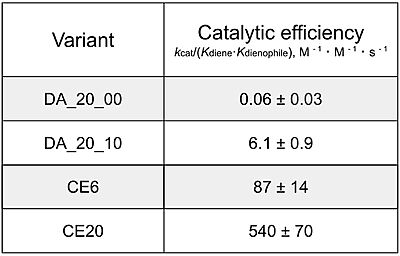
Catalytic efficiency of 4 key generations of Diels-Alderase enzyme. Kinetic data was measured at 25 degrees Celsius, in PBS, at pH 7.4 (cite 3)
Applications
[cite preiswerk] The CE20 model is up to 300 fold more efficient than the first generation model, making it the most efficient Diels-Alderase yet. It surpasses many other biological (antibody) and artificial (ribozyme, metalloenzyme) attempts at catalyzing the Diels-Alder reaction. Even then, the CE20 model has a catalytic efficiency value at least 4 orders of magnitude lower than those seen in other moderately-efficient natural enzymes, demonstrating the innate slowness of the Diels-Alder reaction.
Though the rate of product formation using this enzyme is not significantly different from that found when reactants reflux free in solution, the Diels-Alderase shows a vast improvement in product stereoselectivity. When refluxed in a room temperature aqueous solution containing the necessary substrates, the enzyme catalyzed an over 90% conversion rate, producing only the 3R,4S endo cyclohexane product isomer. By comparison, refluxing the substrates free in solution for a similar duration of time yields a racemic (66:34) mixture of endo and exo products. It is primarily for these stereoselective benefits that this enzyme is valuable for synthetic purposes.
Future improvement of the Diels-Alderase will likely revolve around the improvement of catalytic efficiency, further constriction of the active site, and selective production of varying stereoisomers.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>