User:A. Rahim Zalal/Sandbox 1
From Proteopedia
Histone H2A
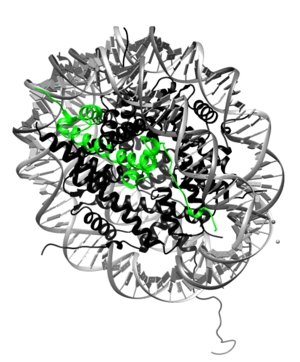
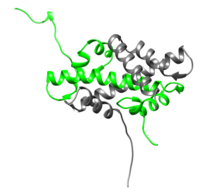
Histone H2A is a subunit of the histone core in the eukaryotic nucleosome. Specifically, H2A molecules form dimers with H2B molecules that interact with tetramers of histones H3 and H4 and another H2A/H2B dimer to form the octameric histone core of the nucleosome.[1] All of the core histone proteins, including H2A, also interact with DNA in ways that allow for DNA compaction.[2]
Contents |
Background
The histone proteins are a family of proteins that allow for the compaction of DNA into functional units known as nucleosomes, which, through certain organized arrangements, form the chromatin fibers in eukaryotic organisms. The histone family includes the core histone families, H2A (shown highlighted on top right), H2B, H3, and H4, as well as the linker histone family, H1. The core histone proteins form the core of the nucleosome, while the linker histones sit on the outside of the nucleosome subunit and assist in interactions with other nucleosomes within the chromatin. To form the core, H3 and H4 form a tetramer that is then joined by two H2A/H2B dimers. This results in two of each core protein forming a histone octamer. The core allows for about 1.7 loops (about 146 bp) of DNA to encircle it.[3] The role of histone proteins in the genetics of an organism also provides another avenue to study the effects of histone protein modifications on the epigenetics of the organism in question.[4]
Structure and Function
| |||||||||||
Sequence
Protein Primary Sequence
The sequence for histone H2A type 1 (human) is presented here[9]:
10 20 30 40 50 60
MSGRGKQGGK ARAKAKTRSS RAGLQFPVGR VHRLLRKGNY AERVGAGAPV YLAAVLEYLT
70 80 90 100 110 120
AEILELAGNA ARDNKKTRII PRHLQLAIRN DEELNKLLGK VTIAQGGVLP NIQAVLLPKK
130
TESHHKAKGK
Coding DNA Sequence
The coding DNA sequence for the H2A type 1 (human)[10]:
ENA|AAA63191|AAA63191.1 Homo sapiens (human) histone H2A.1 : Location:1..393 ATGTCTGGACGTGGAAAGCAAGGCGGCAAAGCTCGGGCAAAAGCTAAAACGCGTTCTTCC AGGGCCGGTCTTCAGTTTCCAGTTGGCCGTGTGCACCGCCTCCTCCGCAAAGGCAACTAC TCCGAACGAGTCGGGGCCGGCGCTCCAGTGTACCTGGCAGCGGTGCTGGAATATCTGACG GCCGAGATCTTAGAGCTAGCTGGCAACGCGGCTCGCGACAATAAGAAGACCCGCATCATC CCGCGCCACCTGCAGCTAGCCATCCGCAACGACGAGGAGCTAAATAAGCTTCTAGGTCGC GTGACCATCGCGCAGGGCGGTGTCCTGCCCAACATCCAGGCCGTATTGCTGCCTAAGAAG ACGGAGAGCCACCATAAGGCCAAGGGCAAGTGA
Some Common Variants
H2A.X
H2A.X is an often-recurring variant of H2A that differs mainly in the C-terminal tail by an addition of 13 residues. The motif some of these residues form allows for the phosphorylation of serine 139 in response to DNA damage. The phosphorylation is the first step of many that lead to DNA repair.[11] In addition to the serine residue, another residue, the C-terminal tyrosine 142 has been shown to exhibit control over cell function with phosphorylation. Specifically, increased phosphorylation of tyrosine 142 seems to be associated with cell apoptosis mechanisms.[12]
H2A.Z
H2A.Z varies even more in primary structure from canonical H2A, but it seems to be more conserved among species. It's variations lead to its role in inhibition of DNA replication/transcription.[13] Specifically, it seems that acetylation of its N-terminus leads to a slightly dissociated nucleosome and allows for transcription.[14]
Alternative Splicing
Alternative splicing of the gene that codes for H2A.Z has been shown to lead to two sub-variants: H2A.Z.1 and H2A.Z.2. The sub-variants only differ in the last amino acid residue, but they could provide insight into how the H2A protein family has evolved.[15]
References
- ↑ Rhodes D. Chromatin structure. The nucleosome core all wrapped up. Nature. 1997 Sep 18;389(6648):231, 233. PMID:9305832 doi:http://dx.doi.org/10.1038/38386
- ↑ Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931-54. PMID:332067 doi:http://dx.doi.org/10.1146/annurev.bi.46.070177.004435
- ↑ 9305837
- ↑ Kass SU, Wolffe AP. DNA methylation, nucleosomes and the inheritance of chromatin structure and function. Novartis Found Symp. 1998;214:22-35; discussion 36-50. PMID:9601010
- ↑ Rhodes D. Chromatin structure. The nucleosome core all wrapped up. Nature. 1997 Sep 18;389(6648):231, 233. PMID:9305832 doi:http://dx.doi.org/10.1038/38386
- ↑ Blank TA, Becker PB. Electrostatic mechanism of nucleosome spacing. J Mol Biol. 1995 Sep 22;252(3):305-13. PMID:7563052 doi:http://dx.doi.org/10.1006/jmbi.1995.0498
- ↑ Rhodes D. Chromatin structure. The nucleosome core all wrapped up. Nature. 1997 Sep 18;389(6648):231, 233. PMID:9305832 doi:http://dx.doi.org/10.1038/38386
- ↑ Biswas M, Voltz K, Smith JC, Langowski J. Role of histone tails in structural stability of the nucleosome. PLoS Comput Biol. 2011 Dec;7(12):e1002279. doi: 10.1371/journal.pcbi.1002279., Epub 2011 Dec 15. PMID:22207822 doi:http://dx.doi.org/10.1371/journal.pcbi.1002279
- ↑ Marzluff WF, Gongidi P, Woods KR, Jin J, Maltais LJ. The human and mouse replication-dependent histone genes. Genomics. 2002 Nov;80(5):487-98. PMID:12408966
- ↑ Albig W, Kardalinou E, Drabent B, Zimmer A, Doenecke D. Isolation and characterization of two human H1 histone genes within clusters of core histone genes. Genomics. 1991 Aug;10(4):940-8. PMID:1916825
- ↑ Bonisch C, Hake SB. Histone H2A variants in nucleosomes and chromatin: more or less stable? Nucleic Acids Res. 2012 Nov;40(21):10719-41. doi: 10.1093/nar/gks865. Epub 2012, Sep 21. PMID:23002134 doi:http://dx.doi.org/10.1093/nar/gks865
- ↑ Singh RK, Gunjan A. Histone tyrosine phosphorylation comes of age. Epigenetics. 2011 Feb;6(2):153-60. Epub 2011 Feb 1. PMID:20935492
- ↑ Singh RK, Gunjan A. Histone tyrosine phosphorylation comes of age. Epigenetics. 2011 Feb;6(2):153-60. Epub 2011 Feb 1. PMID:20935492
- ↑ Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008 Nov 27;456(7221):470-6. doi: 10.1038/nature07509. PMID:18978772 doi:http://dx.doi.org/10.1038/nature07509
- ↑ Moosmann A, Campsteijn C, Jansen PW, Nasrallah C, Raasholm M, Stunnenberg HG, Thompson EM. Histone variant innovation in a rapidly evolving chordate lineage. BMC Evol Biol. 2011 Jul 15;11:208. doi: 10.1186/1471-2148-11-208. PMID:21756361 doi:http://dx.doi.org/10.1186/1471-2148-11-208