User:Caitlin Bell/Sandbox 1
From Proteopedia
Contents |
CHOLERA TOXIN
Introduction
The cholera toxin is released by the pathogen Vibrio cholerae during colonization of the small intestine, which subsequently leads to the Cholera disease. Vibrio organisms are mainly found in saltwater, but there some that live in freshwater. These organisms use glucose for energy, and they use flagella for locomotion.
Cholera is widespread in mainly poverty-stricken areas where food and water environments are unsanitary. After ingestion of Vibrio cholerae, which typically is a result of feces particles in water or food, the cholera toxin is secreted and infects the small intestines, leading to the Cholera disease. Excessive diarrhea and vomiting ensues soon after ingestion, and death can occur within a few hours. Cholera is prevalent in Africa, Asia, and Latin America, leading to 3-5 million cases and 100,000-200,000 deaths every year (1).
|
Structure
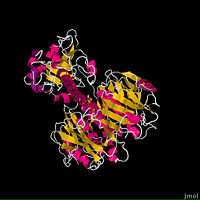
The fundamental structure of the cholera toxin is rather basic. It is a complex of six proteins that are structured into two subunits: A and B. The A subunit contains only one protein and is the only toxic part of the protein. The B subunit contains five proteins and is non-toxic.
The is the most destructive part of the cholera toxin. It can be further broken down into two chains: A1 and A2. These chains are held together through a peptide bond and single disulfide bond through residues Cys187-189. The is entirely non-polar, which allows it to pass through the intestinal membrane and into the cell. Since the cholera toxin is involved in an ADP-ribosylation reaction, the A1 chain has a for NAD+, and when bound, begins the downstream events that ultimately leads to the Cholera disease. Interestingly, the A1 chain is the only catalytic portion of the entire protein. The 's main function is to connect the A1 chain to the B subunit through the B subunit's cylindric pore (2).
The ’s only function is to allow the cholera toxin to enter the intestinal epithelial cells through endocytosis. It accomplishes this from its unique structure. The B subunit contains five alpha helix proteins that are connected together to form a pentagon. Each alpha helix contains a single binding site for the intestinal lining, which is called its (monosialoganglioside binding site) (2).
Mechanism of Action
The overall reaction of the cholera toxin is an ADP-ribosylation reaction, which means that it binds NAD+ and removes an ADP-ribose from it, subsequently transferring it to a G protein.
After the victim has ingested Vibrio cholerea, it travels from the oral cavity to the intestines, where it secretes the cholera toxin within the mucus lining. In the intestines, the B subunit pentamer binds to the lining of the intestinal wall through its five . Once bound, the toxin is engulfed into the cell through endocytosis, and immediately following, the A1 domain is proteolytically cleaved from A2 between residues 182 and 184. Before the A1 chain is considered fully active, the Cys187-Cys189 disulfide bond is reduced, which takes place 20A from the active site of A1. Consequently, A1 is free from the rest of the protein and is able to perform its enzymatic duties.
In order for the ADP-ribosylation reaction to occur, the binding affinity of NAD+ must be increased. This is accomplished through a set of ADP-ribosylation factors (ARFs) that are bound with GTP and are found within the cells. The ARFs bind 15A from the catalytic site and allow the A1 chain to bind NAD+ through its cluster of Arg residues at the , which play an important role in stabilizing the binding through multiple van der waals interactions and hydrogen bonds. However, these Arg residues do not play a catalytic role. The residue of the A1 chain is the essential component in removing the ADP-ribose from NAD+, which is then transferred to the Arg201 residue on the alpha subunit of a stimulatory G protein.
From this ADP-ribosylation reaction, the stimulatory G protein remains in its active state, which means that it is permanently bound to GTP. Thus begins the second messenger downstream events. The active G protein continually activates adenylyl cyclase, which constantly produces the cAMP messenger. The cAMP messenger activates Protein Kinase A, which then uses the energy of hydrolysis of ATP to activate the CFTR-Cl- channel. Constant activation of this ion channel leads to the subsequent massive efflux of water and electrolytes from the cell to the interstitial lumen, which is representative of the symptomatic excessive diarrhea in Cholera. Constant activation of the CFTR-Cl- channel also results in severe metabolic acidosis (4). Because the attached ADP-ribose makes the G protein unable to deactivate, this cycle is continuous.
Treatment
Fortunately, Cholera is relatively easy to treat, provided that the victim reacts quickly and has the resources. “The prompt administration of oral rehydration salts to replace fluids nearly always results in cure. In especially severe cases, intravenous administration of fluids may be required to save the patient’s life” (World Health Organization). However, if untreated, the victim will quickly die as a result of massive loss of fluids.
References
(1) “Cholera”. World Health Organization. June 2010. <http://www.who.int/mediacentre/factsheets/fs107/en/>.
(2) Zhang, Rong-Guang. “The Three-dimensional Crystal Structure of Cholera Toxin”. JMol Biology. Pages 563-573. 1995.
(3) O’Neal, Claire. “Structural Basis for Activation of Cholera Toxin by Human ARF6 GTP”. Science. Volume 309, pages 1093-1096. 12 August 2005.
(4) Bhagavan, NV. “Gastrointestinal Digestion and Absorption”. Essentials of Medical Biochemistry with Clinical Cases. Copyright 2011. Macmillan Company.