Function
is a highly glycosylated multifunctional cell surface receptor that is involved in cell-cell interaction and maintenance of the extracellular matrix.[1] The primary ligand for CD44 is hyaluronan (HA), but it has also been found to interact with other molecules including osteopontin, collagens, and fibronectin. [2] CD44 is a single chain protein containing four distinct regions: the N-terminal HA binding Link domain[3], a flexible variable domain, a transmembrane domain, and an intracellular signaling domain.[1] The is an approximately 100 amino acid domain consisting of two antiparallel β-sheets, two α-helices, and two disulfide bonds.[3] This domain is found in most HA binding proteins, including aggrecan, versican, brevican, and tumor necrosis factor-inducible gene 6.[4]
Evolutionary Conservation
of CD44 residues, as determined by ConSurfDB. Residues proximal to the HA binding site tend to be the most conserved across species while residues that fall outside of the canonical Link domain are the most variable.
Isoforms
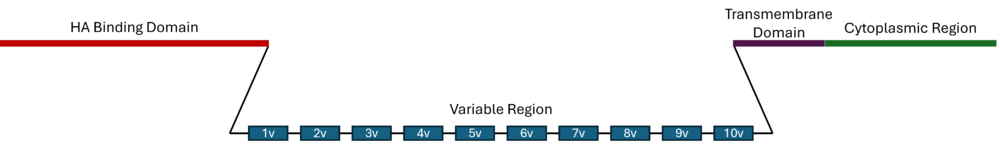
The CD44 gene encodes a total of 20 exons but only exons 1-5 (HA binding domain) and 16-20 (transmembrane domain and cytosolic region) are constitutively expressed.[1][5] Variable exons are termed 1v-10v. Standard CD44 contains no variable regions while variants are termed CD44vX where 'vX' identifies the variable peptides expressed.
Substrate Binding
The HA binding domain of is well characterized. Interactions with tyrosine, alanine, and cysteine residues in the appear to be the most impactful.[6]
Clinical Significance
The Indian blood group system recognizes a handful of CD44 mutants at possible antigens for blood transfusion and organ transplant.[7] Originally only two antigens were recognized, Ina and Inb with 2.38% and 100% frequency respectively in blood donors from Mumbai.[8] Up to three new antigens have been detected in certain populations. All affect a unique single amino acid in the globular extracellular domain but opposite the HA binding cleft.[8]
CD44 has also been shown to be an important co-receptor for the invasion of erythrocytes by Plasmodium falciparum[9], the major causative agent of malaria in humans.[10] Deletion of the CD44 gene from human hematopoietic stem cells results in production of seemingly fully functional erythrocytes that are resistant to Plasmodium falciparum invasion.[9]
Due to its key role in interacting with the extracellular matrix, altered expression of CD44 in tumors is associated with an increased risk of metastasis.[11] For example, benign human prostate cell express CD44v5, but after neoplasia they shift to express soluble CD44 which contains no variable peptides. CD44 expression on vascular endothelial cells contributes to the regulation of angiogenesis and may be leveraged by tumors to increase local blood supply.[1]


