User:Lizun Xin/Mtb BlaC Inhibition
From Proteopedia
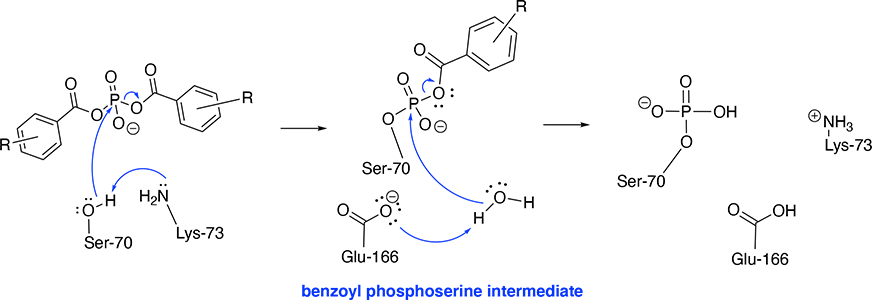
Background on The DiseaseTuberculosis (TB) is a human respiratory disease, which is caused by bacteria: Mycobacterium tuberculosis (Mtb). The most common pathway of transmission for M. tuberculosis is by airborne droplets, for example, coughing and sneezing. M. bovis can be transmitted by animal products, for example uncooked meat and unpasteurised milk. The bacterium can hibernate in the human body for various times, from week to years. TB usually affects the lungs, but in some cases, the infection can spread outside of the lungs (eg. lymphatic system) [1]. As the infection in the lungs develops, patients may experience continuous coughing (sometimes with blood), chest pain and shortage of breath. if the secondary infection affects the immune system, the symptoms often show as fever, sweating and lost weight. When the bacteria are in inactive state, TB does not have the ability to spread, however this proposes a problem for screening the disease. It is estimated that around 30% of the total population may be infected with the bacteria. However the rate of infection is relatively higher in developing countries which may be caused by rather poor medical facility and the knowledge of hygiene. Furthermore, patients with HIV seems to be more vulnerable to the infection, due to their compromised immune system. The standard screening of TB can be done by a chest X-ray. To further diagnose the infection often requires a sample of the patient’s mucus to test if they are TB positive and to differentiate if the strain is drug resistant. The current treatment of TB is most likely to be done with giving combination of antibiotics and the patient mostly likely to be isolated. The only available vaccine available today is the BCG vaccine, which provides around 70% effectiveness Discussion on the Drug ResistanceMulti-drug resistant (MDR) and extensively drug resistant strains (XDR) of M. tuberculosis propose a major threat in public health today. Currently, we lack effective treatment for the MDR and XDR strain Mtb. Existing β-lactam antibiotics can be ineffective, as the XDR Mtb is capable of hydrolysing these drugs. The resistant XDR Mtb is mostly due to the expression of the β-lactamase enzyme, Ambler β-lactamase (BlaC). The BlaC active site is sufficiently large and flexible enable it to accommodate various β-lactams. As the size of BlaC active is relatively large, the desired inhibitor should possess strong hydrophobic characteristics. The method of treatment was proposed, in order to treat XDR Mtb, the β-lactam need to be given in combination with a BlaC active site inhibitor. Therefore, β-lactam inhibitor can inhibit the BlaC active site and allowing β-lactams to inhibit cell wall synthesis and kill the Mtb-causing bacteria. Mechanism of Antibiotic ResistanceAntibiotic resistance is developed though BlaC expression, which to be more specific is a ß-lactamase. The key catalytic active site residues of BlaC can be seen from . Due to the size and flexibility of the BlaC active site, various ß-lactam antibiotics can be bound and hydrolysed. Examples of these antibiotics include: amoxicillin, penicillin, and the cephalosporins. The hydrolysis reaction involves Ser-70, Lys-73 and Glu-166 residues. Initiation: First, the amine group of Lys-73 deprotonates Ser-70 with its lone pair of electrons. Then the reaction starts with an esterification step; the lone pair on oxygen of Ser-70 attacks the β-lactam carbon forming a covalent bond. Meanwhile, the C-N bond in beta-lactam ring is hydrolysed. Hence the βbeta-lactam is destroyed. In , amoxicillin is covalently bounded to BlaC in the active site. Re-activating BlaC: the second step of the mechanism involves the hydrolysis of ester linkage with Lys-73, which allows the BlaC enzyme be reactivated. Glu-166 initiates a hydrolysis reaction to release Ser-70 from the ‘destroyed’ β-lactam with the aid of a water molecule and Ser-70 was re-protonated with additional proton. Thus the enzyme is reactivated and reused. Mechanism of Enzyme InhibitionIn order for the β-lactam antibiotics (e.g. Penicillin or Cephalosporin) to effectively stop bacterial cell wall synthesis, the β-lactamase BlaC active site needs to be inhibited so that it does not destroy the drug first. This can be done by administering a suicide inhibitor (e.g. Clavulanate) along with the beta lactam antibiotic. As the inhibitor binds to the active site of BlaC, it forms a covalent bond with Ser-70. White and colleagues used bis(benzoyl) phosphate inhibitors with BlaC and have shown the inhibition pathway results from phosphorylation of the Ser-70 residue. Some bis(benzoyl) phosphate show strong inhibition of the BlaC active site (R = H, 4-F and 4-OCH3).[2] The mechanism is concerted. The phosphorylation of Ser-70 using these bis(benzoyl) phosphate inhibitors involves the following components: Ser-70, Lys-73, Glu-166 and a water molecule as a final cleavage agent. Initiation: The amine side chain of Lys-73 deprotonates the Ser-70 hydroxy group, which instantaneously attacks the phosphorus atom resulting in the cleavage of one benzoate group. [3] Final Step: Glu-166 deprotonates a water molecule which cleaves the remaining benzoate group. Hence, the . TreatmentsStudying inhibitor potency to the BlaC active site, may allow us to design novel inhibitors. Previous studies suggest Clavulanate is not as effective as predicted when given in combination with cephalosporin. Therefore, better inhibitors for BlaC need to be designed so that the β-lactam antibiotics given in combination with them can more effectively destroy the bacteria. Vaccination ProgrammeThe vaccine effectiveness decreases as the recipient ages. BCG vaccine is not a part of the immune programme in UK and US, as TB is eradicated from those countries. Some other country gives BCG to children, to protect them from possible exposure from the adults. References[1]: M. Jones, R. Fosbery, J. Gregory and D. Taylor; Cambridge International AS and A Level Biology, Chapter 10: infectious diseases-Tuberculosis P209-211, 4th Edition, Cambridge University Press, 2014, ISBN: 978-1-107-63682-8 [2]: D. S. White, C. J. Choy, T. W. Moural, S. E. Martin, J. Wang, S. Gargaro, ChulHee Kang, C. E. Berkman, Bis(benzoyl) phosphate inactivators of beta-lactamase C from Mtb, Bioorganic & Medicinal Chemistry Letters, Volume 29, Issue 16, 2019, Pages 2116-2118, ISSN 0960-894X, Available from: https://doi.org/10.1016/j.bmcl.2019.07.002. [3]: T.Moural, D.White, C.Choy, C.Kang, C.Berkman. Crystal Structure of Phosphoserine BlaC from Mycobacterium tuberculosis Inactivated by Bis(Benzoyl) Phosphate. International Journal of Molecular Sciences. 2019; 20(13):3247. | ||||||||||||