Sandbox Reserved 1239
From Proteopedia
| This Sandbox is Reserved from Jan 17 through June 31, 2017 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, USA. This reservation includes Sandbox Reserved 1225 through Sandbox Reserved 1244. |
To get started:
More help: Help:Editing |
Contents |
Function and Location
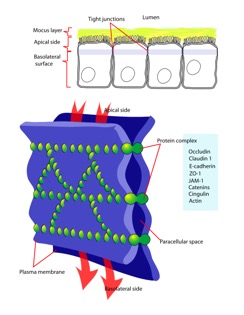
Occludin is one of the proteins absolutely necessary in maintaining tight junctions of cells. Tight junctions preserve the integrity of polarized cells, allowing homeostasis to continue. Polarized cells can be broadly defined as cells that have two dynamically different faces for functionally different purposes. Each face is different in protein and membrane composition. For example, intestinal cells have an apical surface and basolateral surface. The apical surface faces the lumen and intakes material, while the basolateral surface interacts with blood vessels. Proteins and lipids can diffuse laterally and freely through a membrane [1]. However, in cells that are meant to be polarized, it would be catastrophic to have proteins exchanged between apical and basolateral surfaces due to their pivotal roles in homeostasis. Tight junctions restrict lateral diffusion, keeping cell surfaces polarized [2]. Tight junctions, and therefore occluding proteins, heavily influence cell-cell interactions and signal transduction. Cell-cell interactions regulate gene expression and cell differentiation [5].
Structure
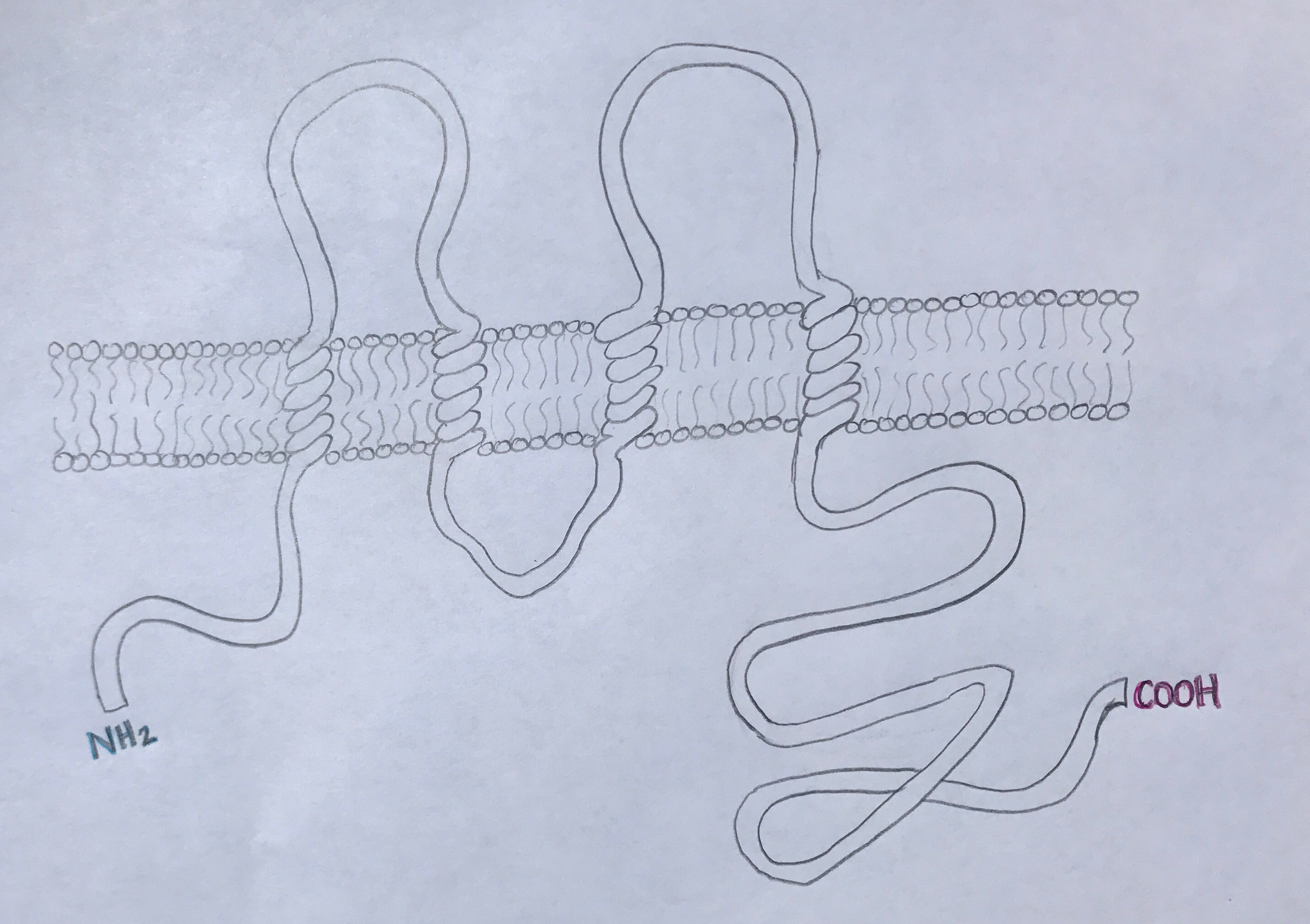
Occludin is approximately 65kDa in length. It was first identified in avian tissue, and was the first integral membrane tight junction protein to be found. Studies have found that occludin structure has 90% homology among mammals [3]. Occludin has four transmembrane alpha-helices, which are responsible for escorting occluding to tight junctions between cells [2]. As can be viewed in the image below, both the amino terminus and carboxyl terminus are located within the cytosol. The C-terminus of occluding is believed to interact with tight junction protein-1 (ZO-1), which is involved in cell-cell signaling [4].
Regulation
As mentioned before, occludin is essential in tight junctions between cells. However, it alone is not sufficient to create a tight junction. Occludin associates with two claudin proteins, which together have been found to be essential to forming a tight junction strand. Other studies found that ZO-1 and occludin together mediate cell adhesion, indicating the pivotal role occludin plays in cell-cell adhesion and tight junctions [6].
It has been found that occluding expression can be regulated by posttranscriptional methods such as gene splicing. Gene splicing also leads to an isoform of occludin that extends the amino terminus by 56 amino acids [2]. Experimental regulation of occludin has helped reveal and confirm possible functions. When the sequence of one of the extracellular loops of occludin was neutralized and disrupted, cell-cell adhesion was interfered with [6].
Occludin is also subject to posttranslational regulation. Proteolytic cleavage of occludin leads to barrier disruption, which often leads to disease and restructure of tissue framework. Occludin can be regulated by reversible phosphorylation. Phosphorylation of key serine, tyrosine, and threonine occludin residues is essential in tight junction construction [2]. Occludin phosphorylation corresponds with vessel barrier dysfunction [7].
Disease
Tight junctions are an essential attribute of homeostatic maintenance within vertebrate physiological systems. Dysregulation of tight junctions is strongly associated with several diseases and pathology [2]. Gene splicing variants contribute greatly contribute to these pathologies. One study found that inducing rat brain endothelium to HIV-1 increased tight junction permeability via alterations in occludin expression [9]. An alternate transcription start site has been identified and associated with increased TNF- signaling. Amongst other things, TNF- is a causative agent in epithelial inflammation in ulcerative colitis, and researchers believe that this is partially due to reduced occludin expression [10].
Occludin dysregulation has also been closely associated with cancers. Cancer itself can also cause tight junction dysregulation as well. One study found that gliomal TGF-2 can lead to reduced expression of occludin. Leukemia has been documented to release factors that degrade occludin, leading to BBB dysfunction and therefore intrusion of leukemia cells into the central nervous system [2, 11].
One of the most studied pathologies that occludin dysregulation interferes with is the blood brain barrier (BBB). The BBB is essential in central nervous system homeostasis due to regulating what substances can pass from the blood to the brain, transport of nutrients and toxins to the brain, and circulation of immune cells. Down-regulation or phosphorylation of occludin has been shown to result in hypoxia, cerebral ischemia, reactive oxygen species, and HIV-1 encephalitis [8]. Preventing occludin down-regulation and disruption is being investigated be a prime pathological therapy.
References
1. Lodish, H., Berk, A., Kaiser, C., Krieger, M., Bretscher, A., Ploegh, H., . . . Scott, M. (2013). (7th ed.). New York, NY: W.H. Freeman and Company.
2. Cummins, P. (2012). Occludin: One Protein, Many Forms. Molecular and Cellular Biology, 32(2), 242-250. doi:10.1128/MCB.06029-11
3. Ando-Akatsuka Y, et al. (1996). Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J. Cell Biology, 133:43–47.
4. Li Y, Fanning AS, Anderson JM, Lavie A. (2005). Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J. Molecular Biology, 352:151–164.
5. Balda, M., & Matter, K. (2009). Tight junctions and the regulation of gene expression. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1788(4), 761-767.
6. Förster, C. (2008). Tight junctions and modulation of barrier function in disease. Histochemistry and Cell Biology, 130: 55. doi:10.1007/s00418-008-0424-9
7. DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA. (2001). Shear stress regulates occludin content and phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 281:H105–H113.
8. Luissint, A.-C., Artus, C., Glacial, F., Ganeshamoorthy, K., & Couraud, P.-O. (2012). Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids and Barriers of the CNS, 9, 23. http://doi.org/10.1186/2045-8118-9-23
9. Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. (N.D.). HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. doi: 10.1002/jnr.10762.
10. Mankertz J, et al. (2002). Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Commun. 298:657–666.
11. Feng S, et al. (2011). Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One 6:e20599.