Sandbox Reserved 1546
From Proteopedia
| This Sandbox is Reserved from May 28 through July 01, 2019 for use in the course Advanced Biochemistry BCHM 4100 taught by Tom Gluick at the Georgia Gwinnett College. This reservation includes Sandbox Reserved 1544 through Sandbox Reserved 1555. |
To get started:
More help: Help:Editing |
Contents |
5H86: HUMAN GCN-5 BOUND TO BUTYRYL-COA
This project was completed by Marianne Javier, Karima MuhummadPoe, and Makayla Yang for Dr. Gluick's Spring 2019 BIOL4100K-01 class at Georgia Gwinnett College.
Project Purpose: This molecule was chosen due to its role as a histone acetyltransferase and its role in transcription regulation [1]. It has a harder time having successful enzymatic activity if the acyl-chain is too long which is the purpose of studying the molecule [1]. These researchers made two different acyl-CoA molecules, propionyl-CoA and butyryl-CoA [1]. The objective of the study is to observe and understand butyryl-CoA, which has a conformation that obstructs the lysine from binding. This molecule was chosen to see how Gcn5L2 chooses which acyl-chain donor to have the highest enzymatic activity.
Function
In order to better understand the function of this 5h86 enzyme, it is important to understand the basic processes of Fatty Acid Degradation. Fatty Acid Degradation is the procedure that fatty acids go through to be broken down into their metabolites and it takes place in the mitochondrial matrix [2]. Intermediates in the fatty acid breakdown are covalently attached to the sulfhydryl group of coenzyme A [2].
Fatty Acid Degradation happens in three steps:
1. Lipolysis and release from adipose tissue: In the initial steps of degradation, fatty acids are stored in the adipocytes [2]. The breakdown of adipocytes is called lipolysis where they are then released into the bloodstream to circulate through the body [2].
2. Activation and transport into the mitochondria: The mitochondria is where fatty acid oxidation occurs which activates fatty acids to be carried to the mitochondria [2]. The enzyme responsible for the catalysis of this step is Fatty-Acyl Coa Synthetase [2]. Malonyl ACP is the activated donor of two carbon units in the elongation step which is operated by the release of CO2 [2].
3. Β-oxidation: Once inside the mitochodria, five steps occur: 1) Activation by ATP, 2) Oxidation by FAD, 3) Hydration, 4) Oxidation by NAD+, and 5) Thyolysis [2]. The final product is Acetyl-CoA which is now able to be able to enter the TCA cycle [2].
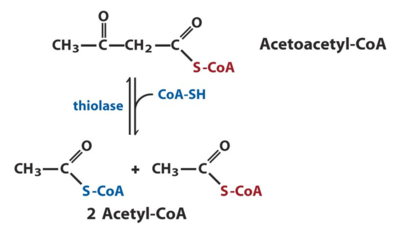
One of the enzymes that have a key role in fatty acid degradation and is the enzyme of study is known as acyl-CoA acetyltransferase or thiolase. Thiolases are enzymes that have key roles in biochemical pathways and can either be degrative or biosynthetic [3]. Degrative thiolases such as 3-ketoacyl-CoA thiolase, plays a role in fatty-acid β-oxidation in peroxisomes and mitochondria and ketone body metabolism in mitochondria, whereas biosynthetic thiolases, like acetoacetyl-CoA thiolase, catalyzes Acetoacetyl-CoA to two Acetyl-CoAs [3].
The specific acetyltransferase of particular interest is known as 5h86 which is a Human Gcn5 bound to butyryl-CoA [1]. Gcn5 is a conserved acetyltransferase that regulates transcription by acetylating the N-terminal tails of histones [1]. To better understand how 5h86 is related to fatty acid degradation, it is crucial to understand how they operate as a histone acetyltransferase (HATs). HATs are enzymes that acetylate conserved lysine amino acids on histone proteins [1]. This occurs by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine [1]. When DNA is wrapped around histones, an acetyl group is transferred to the histones, allowing genes to be turned on and off [1]. In conclusion, histone acetylation contribute to the increase of gene expression [1].
HATs also have a role in transcription regulation and are regulated through phosphorylation [4]. For example, the HAT activity of the CREB-binding protein (CBP) is stimulated by the phosphorylation of a cyclin E/cyclin-dependent kinase 2 [4]. HATs also participate in the genome-wide yield of acetyl groups on histones [4]. Some HATs also target specific promoters through their physical interaction with sequence-specific transcription factors, sectionally modifying histones or transcription components to regulate gene transcription [4].
In all, the function of the 5H68 Human Gcn5 acetyltransferase can be represented through histone acetylation to assist in the activation of transcription [4]. Histone acetylation functions as the main switch that allows the interchange between permissive and repressive chromatin domains during transcription [5]. The histone acetylation-dependent control of gene expression has mechanisms underlying a direct effect on the solidity of nucleosomal arrays and the creation of key sites for the regulatory binding proteins [5]. The enzymes devoted to the addition and removal of acetyl groups are histone acetyltransferases, such as the 5H68 Human Gcn5 acetyltransferase, and deacetylases, in which both enzymes complete acetyl group removal or addition by removing the lysine residues on the N-terminals of histone tails [5].
Structural Highlights & Disease
| |||||||||||
Relevance
Purpose of the Study
It is known that the 5h86 Human Gcn5 enzyme functions as an acetyltransferase that regulates transcription by acetylating the N-terminal tails of histones [1]. The experiment was tested on Gcn5 (Gcn5L2), more specifically the lysine acetylation of this enzyme [1]. This is important to understand because the acyltransferase activity of the Gcn5L2 becomes much weaker with increasing acyl chain length [1]. The researchers were inspired by previous studies that identified a chemically diverse array of lysine acyl modification in vivo, and more specifically - the acyl chain of acetyltransferase specificity in the human Gcn5 [1]. In short, they want to experiment and test which acyl-chain donor had the highest enzymatic activity and to characterize the specificity of the acyl-chain of the human Gcn5, which catalyzes the acetylation of histone peptides much quicker than other methods like propionylation or butyrylation [1]. Through the experiment, it was found that via this method, the active sites of Gcn5 can accommodate longer acyl chains without many structural rearrangements [1].
Experimental Procedure
This experiment was completed in five different parts:
1. Protein Expression and Purification
• A plasmid encoding the His-tagged catalytic domain of human Gcn5L2 under T7 induction was obtained and the protein was expressed and purified [1]. The purified protein was then dialyzed into 20MM and concentrated to 9 mh ml- flash-frozen in liquid nitrogen and stored at -80°C [1].
2. Enzymatic Assays
• Kinetic measurements were used to compare rates of acetylation propionylation and butrylation using a DNTB assay with a few modifications [1].
• The reaction was then incubated for five minutes a 37°C for the remainder of the experiment before adding acyl-CoA and was maintained at that temperature for the remainder of the experiment [1].
• Six data points were collected to find a time frame over which acyl-CoA consumption was linear over time [1]. The reaction was then quenched at the indicated time points by the addition of two volumes of quenching buffer [1].
• After all the samples were collected, one column of 4mM DTNB was dissolved in 100mM sodium phosphate at pH 6.8 [1]. Samples were then moved to a 384-well polystyrene clear-bottom plate and the absorbance at 412 nM was measured in a POLARstar Omega plate reader [1]. The absorbances were converted to concentrations using a standard curve generated by reacting increasing concentrations of CoA with DTNB using an extinction coefficient for 3-thio-6-nitrobenzoate (TNB) of 412 nm = 13,700 M−1 cm−1 [1]. Subsequent reactions were performed in triplicate and quenched after 0.5 minutes (acetyl-CoA), 5 minutes (propionyl-CoA) or 20 minutes (butyryl-CoA) [1].
• All acylation rates were corrected by subtracting the rate of acyl-CoA consumption by Gcn5L2 in the absence of a peptide [1]. Steady-state kinetic titrations varying the acetyl-CoA or butyryl-CoA concentration were then performed with a continuous spectrophotometric assay as previously described [1].
• The acetyl-CoA or butyryl-CoA concentration was varied briefly between 0.25 and 100 µM in the presence of 50 nM hsGcn5L2 and 300 µM histone H3 peptide [1]. Reactions were performed in a total volume of 50 µl at 37°C in 384-well plates (Greiner Bio-One) and were initiated with the acyl-CoA [1]. The absorbance at 340 nm was monitored continuously using a POLARstar Omega plate reader (BMG Labtech) for 5–20 min and converted into the molar concentration of NADH using Beer's law, assuming ∊340 nm = 6220 M−1 cm−1 [1]. For controlled variables, rate measurements were performed at each concentration of acyl-CoA in the absence of peptide [1]. Each measurement was performed in triplicate, and reaction velocities in the presence of peptide were blanked by the rate of reaction in the absence of peptide [1].
• Blanked rates were normalized to enzyme concentration, plotted as a function of substrate concentration, and fitted to the Michaelis–Menten equation using nonlinear least-squares regression in GraphPad Prism 5 [1]. Butyryl-CoA inhibition measurements were also performed with the enzyme-coupled assay [1]. Reaction velocities were measured in the presence of 0.5–10 µM acetyl-CoA and 50 nM hsGcn5L2 with increasing concentrations of butyryl-CoA (0, 50, 100 or 300 µM) [1]. Under these conditions, the consumption of butyryl-CoA by hsGcn5L2 was undetectable by the same assay [1]. Blanked rates were normalized to enzyme concentration and the resulting curves were globally fitted to a competitive-inhibition model in GraphPad Prism 5 [1].
3. HAT-domain Crystallization
• Propionyl-CoA and butyryl-CoA were diluted in 20 mM HEPES pH 7.5 and stored at −20°C at a concentration of 20 mM as calculated using an absorbance of 260 nm = 16,400 M−1 cm−1 [1]. Purified human Gcn5L2 (amino acids 497–662) were mixed with each acyl-CoA to produce a final concentration of 1.6 mM acyl-CoA and 7.9 mg ml−1 protein [1]. NaCl was added for a final concentration of 125 mM from a 5 M stock, and the resulting mixture was incubated on ice for 30 minutes [1]. Both complexes were crystallized using hanging-drop vapor diffusion by mixing 1 µl protein–acyl-CoA complex solution with 1 µl well solution [1]. Human Gcn5L2 bound to propionyl-CoA was crystallized in 20%(v/v) ethanol, 100 mM Tris pH 9.0. Human Gcn5L2 bound to butyryl-CoA was crystallized in 10%(v/v) 2-propanol, 3% glycerol, 100 mM HEPES pH 7.8. [1]
• Crystals were then cryoprotected by soaking in a well solution supplemented with 9% sucrose, 4% glucose, 8% ethylene glycol and 8% glycerol [1]. Prior to data collection, crystals were flash-cooled in a liquid-nitrogen stream [1].
4. Data Collection and Processing
• This step composed of data collection and analysis from the experiment [1].
5. PDB Accession Codes
• This step composed of data entry into the Protein Data Bank (PDB) [1].
Experimental Results
The findings of this experiment included:
• Gcn5 is a weak acetyltransferase [1]
• Discovery of the structures of a Hcn5L2 bound to propionyl-CoA and butyryl-CoA [1]
• Human Gcn5L2 efficiently acetylates and propionylates peptides, while its butyrylating activity is nearly undetectable [1]
• Construction of a model of the ternary complex with peptide and CoA [1]
• Butyryl-CoA is a competitive inhibitor of acetylation by human Gcn5 [1]
• Gcn5L2 propionylates histone peptides approximately ninefold more slowly and butyrylates peptides nearly 400-fold more slowly compared with its acetyltransferase activity [1]
• Based on these relative rate measurements, Gcn5L2 is unlikely to contribute significantly to lysine butyrylation in vivo but may be capable of catalyzing lysine propionylation under physiological conditions [1]
Overall Relevance, Conclusions, and Future Steps
The findings of the experiment helps future researchers to be able to determine crystal structures that are able to describe how Gcn5 accommodates propionyl-CoA in the active site [1]. The results of the experiment have also provided a structural mechanism that explains how the Gcn5 discriminates between different acyl-CoA molecules [1]. Further data indicate that butyryl-CoA is more of a competitive inhibitor than acetyl-CoA for human Gcn5 [1]. This then raises the question as to whether fluctuating the levels of acyl-CoA molecules in cells may regulate the activity of Gcn5, which may possibly be tested in future experimentations.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 1.33 1.34 1.35 1.36 1.37 1.38 1.39 1.40 1.41 1.42 1.43 1.44 1.45 1.46 1.47 1.48 1.49 1.50 1.51 1.52 1.53 1.54 1.55 1.56 Ringel AE, Wolberger C. Structural basis for acyl-group discrimination by human Gcn5L2. Acta Crystallogr D Struct Biol. 2016 Jul 1;72(Pt 7):841-8. doi:, 10.1107/S2059798316007907. Epub 2016 Jun 23. PMID:27377381 doi:http://dx.doi.org/10.1107/S2059798316007907
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 22.4, Fatty Acids Are Synthesized and Degraded by Different Pathways. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22554/
- ↑ 3.0 3.1 Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21154/
- ↑ 4.0 4.1 4.2 4.3 4.4 Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003 Oct;4(10):944-7. doi: 10.1038/sj.embor.embor941. PMID:14528264 doi:http://dx.doi.org/10.1038/sj.embor.embor941
- ↑ 5.0 5.1 5.2 Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. 2005 Jun;83(3):344-53. doi: 10.1139/o05-041. PMID:15959560 doi:http://dx.doi.org/10.1139/o05-041
- ↑ 6.0 6.1 6.2 6.3 Ott M, Schnolzer M, Garnica J, Fischle W, Emiliani S, Rackwitz HR, Verdin E. Acetylation of the HIV-1 Tat protein by p300 is important for its transcriptional activity. Curr Biol. 1999 Dec 16-30;9(24):1489-92. PMID:10607594