Sandbox Reserved 459
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
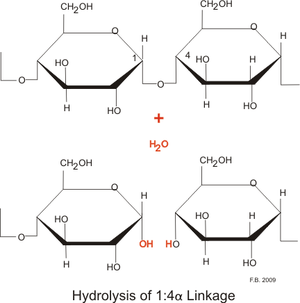
AmylaseIntroductionAmylase is categorized in a class of enzymes known as hydrolases. Hydrolases are enzymes which use water to cleave chemical bonds, usually dividing a large molecule into two smaller molecules. Examples of common hydrolases include esterases, proteases, glycosidases, nucleosidases, and lipases. Hydrolases carry out important degradative reactions in the body. During digestion, lipases hydrolyze lipids and proteases convert protein to amino acids. Hydrolases cleave large molecules into fragments used for synthesis , the excretion of waste materials, or as sources of carbon for the production of energy. [1] Amylase is an enzyme that catalyses the breakdown of starch into sugars. Amylase is present in human saliva, where it begins the chemical process of digestion. Food that contains much starch but little sugar, such as rice and potato, taste slightly sweet as they are chewed because amylase turns some of their starch into sugar in the mouth. The pancreas also makes amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. As diastase, amylase was the first enzyme to be discovered and isolated. Amylase in all organisms has the function of breaking down complex carbohydrates.
StructureTo examine the crystal structure of human salivary amylase, X-ray crystallography was used with a resolution of 1.60 Å. [2] The active site of alpha-amylase contains a trio of acidic groups that do most of the work. Ca2+ is a common for alpha amylase.The Ca2+ ion is bound to Asnl00, Arg158, Asp167, His201 and three water molecules. The Cl- ion is bound to Arg195, Asn298 and Arg337 and one water molecule. The highly mobile glycine-rich loop 304-310 may act as a gateway for substrate binding and be involved in a `trap-release' mechanism in the hydrolysis of substrates. Strategic placement of calcium and chloride ions, as well as histidine and tryptophan residues may play a role in differentiating between the glycone and aglycone ends of the polysaccharide substrates. Salivary amylase also possesses a suitable site for binding to enamel surfaces and provides potential sites for the binding of bacterial adhesins. . Salivary amylase folds into a multidomain structure consisting of three domains, A, B and C. Domain A has a (a/b)8- barrel structure, domain B has no definite topology and domain C has a Greek-key barrel structure. Circular dichroism spectroscopic data revealed the native alpha-amylase to contain 25% , 21% , and 54% random coils. [3] It is generally assumed that in proteins hydrophobic residues are not favorable at solvent-exposed sites, and that amino acid substitutions on the surface have little effect on protein thermostability. Contrary to these assumptions, hyperthermostable variants of alpha amylase have been identified that result from the incorporation of at the surface. [4]
Mechanism of ActionHydrolysis of starch or oligosaccharides by mammalian amylases, in general, results in maltose as the leaving group. The active site of these amylases harbors three aromatic residues Trp59, Tyr62, and Tyr151, which provide stacking interactions to the bound glucose moieties. The hydrolysis is catalyzed by three carboxyl groups and its starts from a water nucleophilic attack and opening of the glucose ring in the catalytic center rather than from protonation of the glycosidic oxygen. One of the carboxylic acids in the active site acts as the catalytic nucleophile during the formation of the intermediate. A second carboxylic acid operates as the acid/base catalyst, supporting the stabilization of the transition states during the hydrolysis. The chain length of the oligosaccharide has an impact on both the rate of the hydrolytic degradation and the product pattern produced by a-amylase. Oligomers with more than six glucose units are hydrolyzed more rapidly, because they are more akin to the natural substrates of a-amylase. [5]
Medical Implications or Possible Applications
Amylase InhibitorsAmylase inhibitors are also known as starch blockers because they contain substances that prevent dietary starches from being absorbed by the body.
References
|