Sandbox Reserved 472
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Introduction
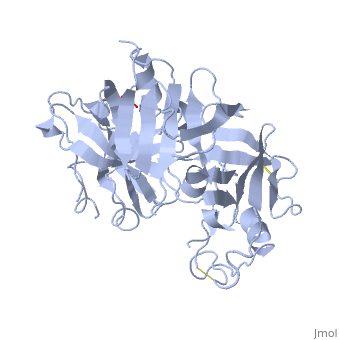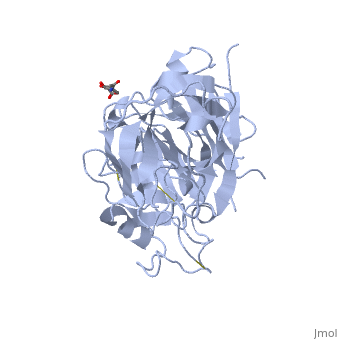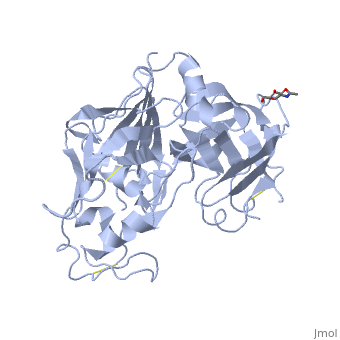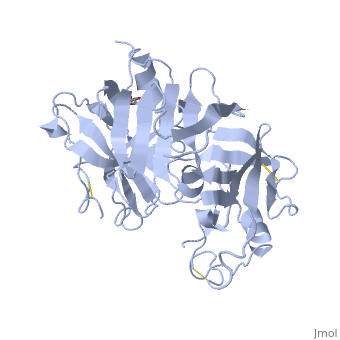
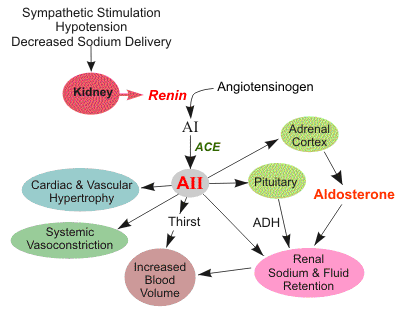
Renin was first discovered in 1898 by physiologist Robert Tigerstedt and his medical student Bergman. It is an aspartyl-protease that plays a major role in the physiological rennin-angiotensin-aldosterone system; therefore indirectly involved in homeostatic control of salt, volume and blood pressure. The enzymatic reaction of renin is the cleavage of Lue bond in angiotensinogen to make angiotensin. In the human body, renin is synthesized by juxtaglomerular epitheloid cells proceeding stimulation by the prostaglandins and sympathetic nerves. Secretion of renin is controlled by three secondary messengers: cAMP, cGMP, and cytosolic Calcium. Studies have shown that the protease is inhibited by angiotensin II, high blood pressure, salt and volume overload; all through negative feedback loops. Once produced, renin enters the body’s circulation in renal system through afferent arterioles. Expression of the renin gene in these cells have been heavily studied in humans and mice and mapped to chromosome 1. StructureAs displayed to the right of the page, a folded recombinant human renin molecule consists of two lobes. Located between these lobes is the at the bottom of the cleft consisting of two catalytic aspartic residues. At this site, the pro-segment of the inactive form of renin forms a plug thereby hindering substrate binding. The of the protease include seven helices containing 35 residues and 28 strands containing the remaining 136 residues. X-ray diffraction was the method of choice used to solve the structure. Mechanism of ActionImmediately after translation, a preprorenin enters the endoplasmic reticulum complex of a juxtaglomerular cell. "Pre" portion of the inactivated protease is then cleaved off upon the entrance of cisterns and proceeds to make its way to the golgi apparatus for glycosylation and tagging for further processing and eventual secretion through the regulated pathway. Trans-golgi, in comparison, release clear vesicles of prorenin that are secreted through the constitutive pathway as inactive renin. Prorenin tagged for the regulated pathway enters in a protogranule. This complex becomes one molecule, cleaving the "pro" segment with the aid of a proconvertase enzyme to become active. The activated renin is finally released from the cell through regulated exocystosis. Exocytosis is controlled by three intracellular secondary messengers, namely cAMP, cGMP and cytosolic Calcium. However, cAMP signaling is currently the central factor of physiological renin secretion.
Medical Relevance for HumansToo much secretion of renin leads to vasoconstriction of blood vessels as well as retention of sodium and water. All these combined are precursors of developing hypertension and heart failure; thereby making the control of the renin-angiotensin-alderstone system a major study in pharmacology. Renin inhibitors, namely the angiotensin converting enzyme inhibitor (ACE inhibitor), have been identified and used to treat hypertension based on their specific ligand binding to ACE. Other inhibitors such as angiotensin-receptor blockers and direct renin inhibitors are currently being studied for several physiological effects before being approved for the public. However, direct renin blockers seem to be better than the others in that they display prolonged plasma as well as extended tissue half life. References1. Schweda, Frank, Ulla Friis, Ole Skott, and Armin Kurtz. "Renin Release." Physiology 22.5 (2007): 310-319. Print. 2. Castrop, Hayo, Klaus Hocherl, Amin Kurtz, Frank Schweda, Vladimir Todorov, and Charlotte Wagner. "Physiology of Kidney Renin." Physiology 90.2 (2010): 607-673. Web. 3. Sica, Domenic A. "The Pharmacology of Agents That Interfere with Renin-Angiotensin System Activity." Journal of Renin-Angiotensin-Aldosterone System 7.4 (2006): 247-250. Web. 4.Sampoornam, Balakrishnan, Khashim Zenith, and Samuel Shila. "Characterization and Structural Annotation of Renin and Angiotensin Converting Enzymes." BIOMIRROR (2010): N. pag. Web. <http://www.bmjournal.in/index.php?option=com_content&view=article&id=139:characterization-and-structural-annotation-of-renin-and-angiotensin-converting-enzymes&catid=50:july&Itemid=142>. 5.Sielecki, A.R., Hayakawa, K., Fujinaga, M., Murphy, M.E., Fraser, M., Muir, A.K., Carilli, C.T., Lewicki, J.A., Baxter, J.D., James, M.N. "Structure of Recombinant Human Renin, a Target for Cardiovascular-active Drugs, at 2.5 a Resolution." Science 243(1989): 1346-1351. http://www.pdb.org/pdb/explore/explore.do?structureId=2REN. |