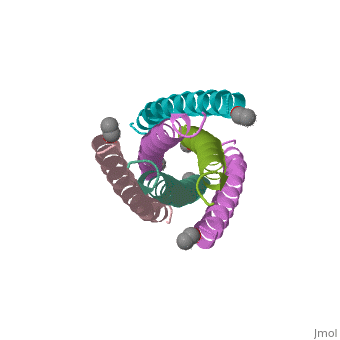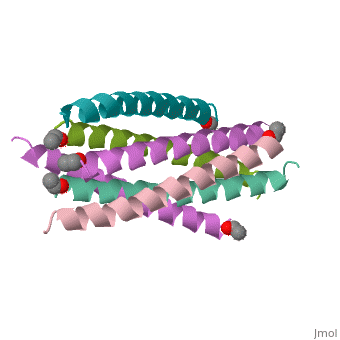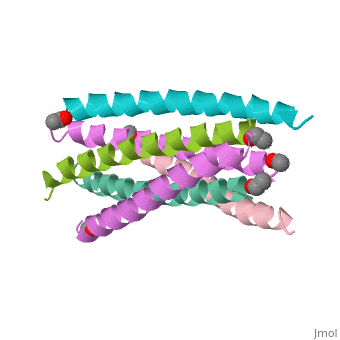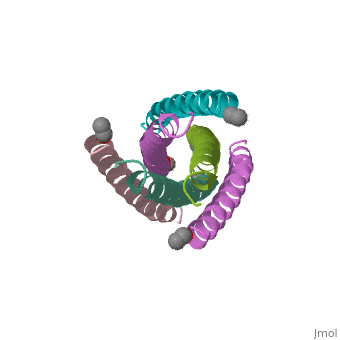Sandbox Reserved 593
From Proteopedia
| This Sandbox is Reserved from Feb 1, 2013, through May 10, 2013 for use in the course "Biochemistry" taught by Irma Santoro at the Reinhardt University. This reservation includes Sandbox Reserved 591 through Sandbox Reserved 599. |
To get started:
More help: Help:Editing |
Contents |
Background
Glycoprotein 41, also known as gp41, is a subunit of the envelope protein (Env) found on several retroviruses, most notably HIV. Env is originally synthesized as gp160, which is eventually cleaved into gp41 and gp120. These subunits are noncovalently bonded together and are critical for viral infection [1]. Gp41 is a transmembrane protein that mediates fusion between HIV-1 and the host cell. Gp41 is one of the major targets for the immune response in infected individuals, and its crucial role in HIV-1 infection has prompted the development of various treatments aiming to inhibit its function[2].
Human Immunodeficiency Virus (HIV) is a virus that destroys the CD4 cells of the body’s immune system. It is transmitted when infected body fluids such as semen or blood either come into contact with either a mucous membrane or damaged tissue or are injected into the blood stream. HIV infection progresses through different stages: acute infection, clinical latency, and AIDS. Two to four weeks after a person becomes infected with HIV they can enter into the acute infection stage of infection where CD4 cell count dramatically falls as the number of HIV particles spikes. Eventually, CD4 cell levels stabilize and the body enters into the clinical latency stage, in which HIV reproduces at a very low rate and has little or no real affect on the body. Acquired Immunodeficiency Syndrome (AIDS) is the stage of HIV infection where the virus drastically damages the immune system leaving the body vulnerable to infections[3].
Structure
|
![Figure 1: A) Ribbon Diagram of N36/C34 Complex of gp41. The N36 helices (gray) shown as a trimeric coiled coil, and the C34 helices (blue) shown wrapped around this coil. B) N36 coils (gray) shown as molecular surface with C34 helices (blue) packed against conserved groove. Bottom of coil has large cavity that becomes a binding pocket for C34 residues (red).[Figure from (Chan et al., 1998)]](/wiki/images/thumb/9/9d/Picforproteo.png/250px-Picforproteo.png)
Gp41 is composed of 345 amino acid residues. It consists of three domains: the ectodomain, transmembrane domain, and cytoplasmic domain. The ectodomain of gp41 contains three important functional regions that function in gp41 activity, namely the fusion peptide, and both the N-terminal and C-terminal heptad repeats[5]. Protein dissection studies show that the two hydrophobic heptad repeat regions within the gp41 structure form a helical trimer made up of antiparallel dimers, and crystallographic analysis confirm that the gp41 core is a bundle comprised of N (amino terminal) and C (carboxyl terminal) helices[6]. The C-terminal of gp41 is the transmembrane segment that is found in the viral membrane, and the N-terminal of gp41 is found outside the viral membrane beneath the gp120 proteins.
The gp41 core is a N36/C34 Complex (Figure 1). The N36 peptides form the three central helices, which are arranged in a trimeric-coiled coil. The C34 peptides form three outer helices. These C34 helices pack into highly conserved hydrophobic grooves on the surface of the N36 coiled coil.
Function
Gp41 and gp120 work together to mediate cell binding and fusion. Gp120 directs cell recognition and cell binding through interactions with CD4, a glycoprotein found on immune cells, and a chemokine receptor. The chemokine receptor (either CCR5 or CXCR4) is the primary receptor that binds gp120 to the target cell. CD4 serves more to concentrate the virus at the surface of the cell[7]. Gp120 binding triggers conformational changes in gp41 that promote fusion between the virus and the target cell resulting in the release of viral contents into the host cell[8].
When gp120 binds to CD4 and the chemokine coreceptor molecules, the V1/V2 loops of its structure rotate outwards and expose the N-terminal of gp41, which include the fusion protein[9]. Upon gp120 binding, gp41 enters into the fusion active state and mediates cell fusion[10]. The conformational changes that gp41 undergoes can be sorted into different stages of conformational states: prefusion, prehairpin, and post fusion. Prefusion is where the protein exists in the transmembrane conformation. After gp120 binding, gp41 changes to the prehairpin conformation, where it extends into the host cell. Further conformational changes lead to the post fusion state, where gp41 collapses into trimer hairpins and completely enters the host cell causing fusion (Figure 2).
![Figure 2: Model of gp41 mediated fusion. Gp120 binding exposes N-terminal (NHR) containing the fusion protein (fp), which then extends into target cell. Gp41 undergoes conformational changes that leads to fusion of the membranes. [Figure from (Yu et al., 1999)]](/wiki/images/thumb/1/19/Function.png/350px-Function.png)
Clinical Relevance
There is currently no cure for HIV; however there are various antiretroviral therapies used to treat the disease and prolong the lives of afflicted individuals. There are four classes of drugs approved by the FDA: reverse transcriptase inhibitors (RTI), protease inhibitors (PI), integrase inhibitors, and fusion/entry inhibitors. HIV fusion/entry inhibitors can target the early steps of the viral replication cycle, where envelope proteins such as gp41 help to fuse the virus to its host T-cell. These inhibitors have also been used to help treat patients who fail to respond to RTI and PI therapies[12].
Video microscopy and fluorescent dye bis-AND have been used to monitor the conformational changes undergone by gp41. These studies show that Gp41 enters the prehairpin state within four minutes of gp120 binding and remains in that conformation for approximately fifteen minutes. In this time, the N-terminal is exposed; the cavities of the coiled coil are attractive targets for the development of new small-molecule inhibitors of HIV infection. Binding to the coil could potentially prevent gp41 from undergoing any further conformational changes and therefore prevent fusion[13]. Peptides that overlap or are derived from the N and C peptide regions of gp41 are able to bind to gp41 and have been shown to have potent anti-viral activity[14].
In 2003, the FDA approved T-20, enfuvirtide[15], as the first HIV fusion inhibitor for treatment of HIV/AIDS. T-20 cannot bind to the hydrophobic pocket formed by the N36/C34 complex and consequently has low anti-HIV activity. However, use of T-20 along with other antiretroviral therapies has resulted in strong synergistic effects. New HIV fusion inhibitors are being developed with the ability to bind to the hydrophobic pocket[16].
One of the hindrances to developing a gp41 inhibitor is the highly dynamic nature of the HIV glycoproteins. The virus constantly changes the amino acid sequences for both gp120 and gp41. These changes have very little effect on the actual protein function. Gp41’s highly helical nature makes it very stable, so changes to the amino acid residues does not denature the protein. A study that looked at the differences between wild type gp41 and various cysteine-alanine mutants showed minimal structural alterations even with two or three introduced mutations that did not affect the biochemical properties of the protein[17]. This makes developing an inhibitor that can consistently bind to gp41 more difficult; however, the potential of fusion inhibition as a viable means of antiretroviral therapy for those afflicted with HIV-1 is increasing as more is learned about the structure of gp41.
References
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Flores N, Harel M. 2013. Hiv env proteins [Internet]. Proteopedia; [2013 Mar 14, cited 2013 Apr 5]. Available from: http://www.proteopedia.org/wiki/index.php/Hiv_env_proteins
- ↑ Centers for Disease Control and Prevention (US). HIV/AIDS [Internet]. Atlanta, GA: CDC [updated 2013 Apr 22; cited 2013 Apr 23]. Available from: http://www.cdc.gov/flu/avian/gen-info/avian-flu-humans.htm
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Yu F, Lu L, Du L, Zhu X, Debnath AK, Jiang S. Approaches for identification of HIV-1 entry inhibitors targeting gp41 pocket. Viruses. 2013 Jan 11;5(1):127-49. doi: 10.3390/v5010127. PMID:23344560 doi:10.3390/v5010127
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Littman DR. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998 May 29;93(5):677-80. PMID:9630212
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Flores N, Harel M. 2013. Hiv env proteins [Internet]. Proteopedia; [2013 Mar 14, cited 2013 Apr 5]. Available from: http://www.proteopedia.org/wiki/index.php/Hiv_env_proteins
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Yu F, Lu L, Du L, Zhu X, Debnath AK, Jiang S. Approaches for identification of HIV-1 entry inhibitors targeting gp41 pocket. Viruses. 2013 Jan 11;5(1):127-49. doi: 10.3390/v5010127. PMID:23344560 doi:10.3390/v5010127
- ↑ Yu F, Lu L, Du L, Zhu X, Debnath AK, Jiang S. Approaches for identification of HIV-1 entry inhibitors targeting gp41 pocket. Viruses. 2013 Jan 11;5(1):127-49. doi: 10.3390/v5010127. PMID:23344560 doi:10.3390/v5010127
- ↑ Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998 May 29;93(5):681-4. PMID:9630213
- ↑ Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997 Apr 18;89(2):263-73. PMID:9108481
- ↑ Medilexicon International Ltd. Fuzeon (enfuvirtide): Drug Information [Internet]. Bexhill-on-Sea, UK: Medilexicon. [2013, cited 2013 Apr 23]. Available from: http://www.medilexicon.com/drugs/fuzeon.php
- ↑ Yu F, Lu L, Du L, Zhu X, Debnath AK, Jiang S. Approaches for identification of HIV-1 entry inhibitors targeting gp41 pocket. Viruses. 2013 Jan 11;5(1):127-49. doi: 10.3390/v5010127. PMID:23344560 doi:10.3390/v5010127
- ↑ Krell T, Greco F, Engel O, Dubayle J, Dubayle J, Kennel A, Charloteaux B, Brasseur R, Chevalier M, Sodoyer R, El Habib R. HIV-1 gp41 and gp160 are hyperthermostable proteins in a mesophilic environment. Characterization of gp41 mutants. Eur J Biochem. 2004 Apr;271(8):1566-79. PMID:15066182 doi:10.1111/j.1432-1033.2004.04068.x