You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Function and Structural highlights
HSH155HEAT is a core splicing factor that is associated with the U2 small nuclear ribonucleoproteins complex (U2 snRNA), a component of the spliceosome of Saccharomyces cerevisiae. The HSH155 is a major component to the splicing of the pre-mRNA, this protein binds with the pre-mRNA upstream of the intron branching site no matter the sequence of this RNA, anchoring the U2 snRNA to the pre-mRNA[3].
The HSH155 is composed by a single peptide, the structure of the region closer to the N-terminal is still unresolved but the rest of this protein is composed by a series of alpha helices in tandem, denominated HEAT repeat, this structure is characterized by repetitions of two alpha helices linked by a short loop, forming a solenoid form that resembles the letter “C” [4].
This structure is essential to the function of this protein, allowing ligants to interact with the innermost part of this molecule and alter its opening, being capable of small regulations in its capacity to interact with the pre-RNAm and other molecules of the U2 snRNA. Besides that, this structure has two main states: Open and Closed. This can be regulated and permits the interaction and the fixation of the pre-RNAm, and this process allows the formation of the A complex during the splicing, the closing of the HSH155HEAT that permits the assemble of the A complex[5]. The image bellow shows the difference between the open and closed strucutres and how it affects the interaction with the pre-mRNA.
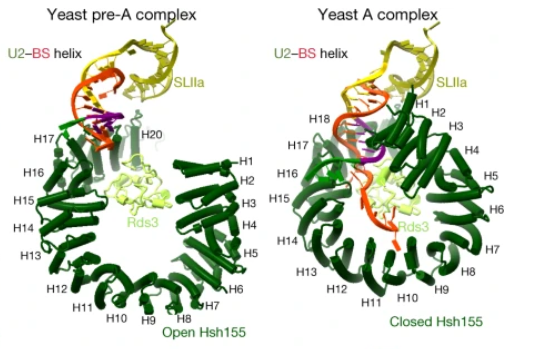
In the box at the right, it is possible to see its in a space-fill model obtained by electron microscopy at 9 Å resolution. However, to cover some important aspects of the structure and function of the molecule, it is particularly useful to represent its .
This previous representation shows that the alpha helices are a major component of this protein, in you can also see that, now with red to represent alpha helices and blue for the loops. You can also see a representation from the (blue represents the N-terminus, while red the C-terminus).
U2 snRNA
The HSH155HEAT is a major component of the , this complex is present in almost all of the eukaryotes already studied. This means that similar components like the HSH155HEAT are also present. In humans this protein is the SF3B1 (Splicing factor 3B subunit 1). Both have similar structure and the presence of the HEAT repeats on the C terminal part of the protein[6].
In eukaryotes the U2 snRNA is essential for the assembly of the Pre-A complex, because of the ability to recognize and bind to the intron on mRNA primary transcripts. This highly dependent on the HSH155HEAT, but the U2 snRNA also has been proposed to have a catalytic purpose on the splicing. Although this function is not a direct result of the interaction of the RNAm with the HSH155HEAT, this protein is also involved by regulating the attachment and the movement of associated proteins in this complex when it shifts between the open and close states[7].
The U2 snRNA can vary vastly between all eukaryotes, having different lengths and sequences according to the species. But that is also some very conservated parts of this complex and the HEAT repeats are one of them. This fact indicates how important this protein and this component are to the process of splicing[8].
In Saccharomyces cerevisiae, the U2 snRNA part of the Pre-A complex contains 19 unique protein chains. You can also see the representation of the patterns for this complex with different colors for each chain, and you can see the HSH155HEAT in blue while the rest of the structure is colored in red.
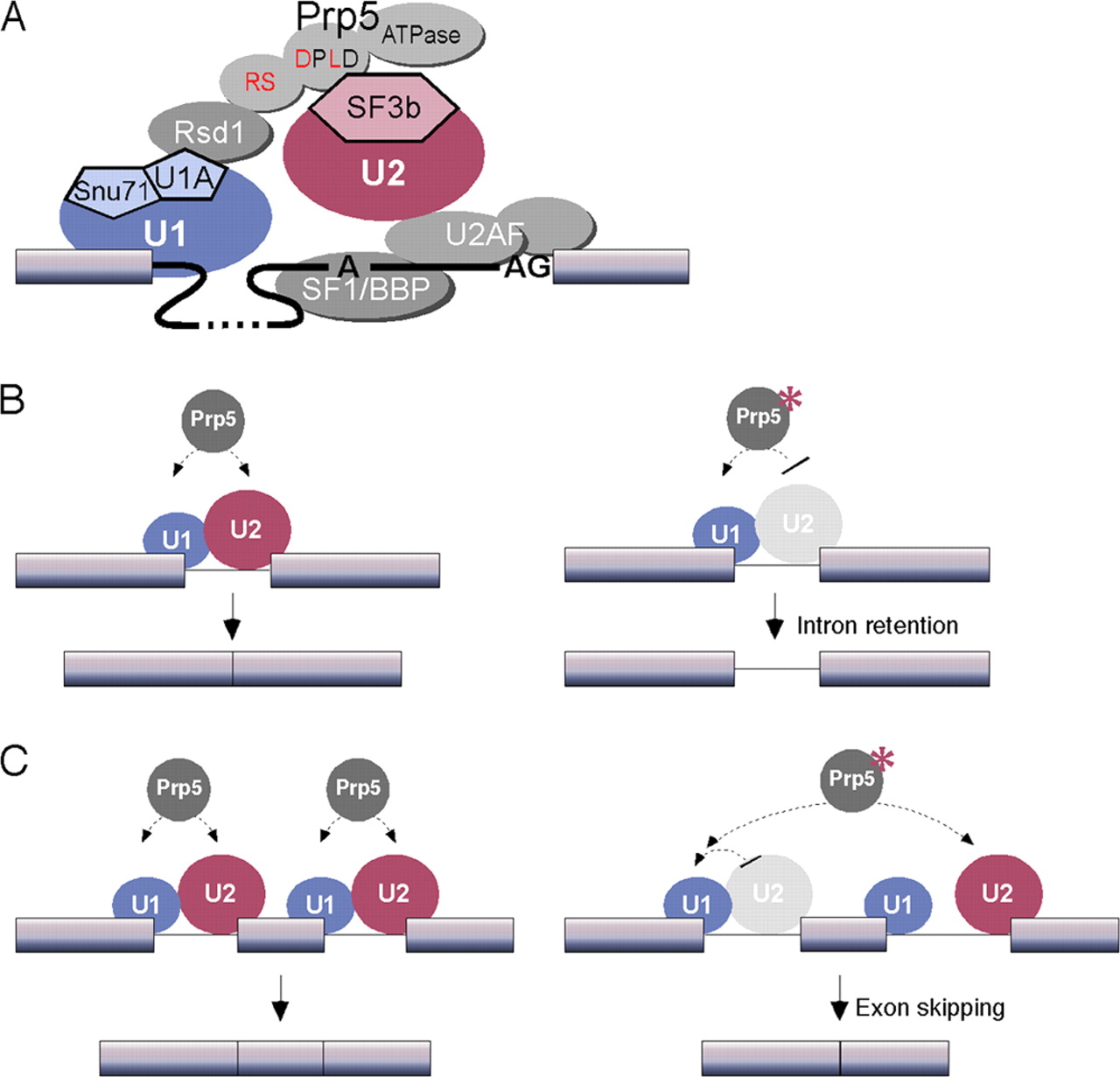
While the U2 snRNA identifies and binds with the branching point of the intron, the U1 snRNA identifies and binds with the 5’ splicing site. And together these two form the spliceosome A complex. This is one of the first steps needed to begin the process of splicing in eukaryotic cells[9]. The image bellow show a simplified example of how the U1 and U2 complexes interact with the pre-mRNA before the splicing.
Diseases
In humans, mutations in the SF3B1 protein are linked to some diseases like myelodysplastic syndrome, breast cancer and chronic lymphocytic leukemia. In a recent study 9.7% of patients with chronic lymphocytic leukemia had mutations in the SF3B1, and although mutations in this protein don’t account for all of the cases of these conditions, they are also linked with an increased progression rate and severity in these patients[10].


